Comparative effectiveness of mRNA-1273 and BNT162b2 COVID-19 vaccines in immunocompromised individuals: a systematic review and meta-analysis using the GRADE framework
- PMID: 37771594
- PMCID: PMC10523015
- DOI: 10.3389/fimmu.2023.1204831
Comparative effectiveness of mRNA-1273 and BNT162b2 COVID-19 vaccines in immunocompromised individuals: a systematic review and meta-analysis using the GRADE framework
Abstract
Introduction: Despite representing only 3% of the US population, immunocompromised (IC) individuals account for nearly half of the COVID-19 breakthrough hospitalizations. IC individuals generate a lower immune response after vaccination in general, and the US CDC recommended a third dose of either mRNA-1273 or BNT162b2 COVID-19 vaccines as part of their primary series. Influenza vaccine trials have shown that increasing dosage could improve effectiveness in IC populations. The objective of this systematic literature review and pairwise meta-analysis was to evaluate the clinical effectiveness of mRNA-1273 (50 or 100 mcg/dose) vs BNT162b2 (30 mcg/dose) in IC populations using the GRADE framework.
Methods: The systematic literature search was conducted in the World Health Organization COVID-19 Research Database. Studies were included in the pairwise meta-analysis if they reported comparisons of mRNA-1273 and BNT162b2 in IC individuals ≥18 years of age; outcomes of interest were symptomatic, laboratory-confirmed SARS-CoV-2 infection, SARS-CoV-2 infection, severe SARS-CoV-2 infection, hospitalization due to COVID-19, and mortality due to COVID-19. Risk ratios (RR) were pooled across studies using random-effects meta-analysis models. Outcomes were also analyzed in subgroups of patients with cancer, autoimmune disease, and solid organ transplant. Risk of bias was assessed using the Newcastle-Ottawa Scale for observational studies. Evidence was evaluated using the GRADE framework.
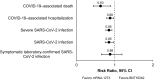
Results: Overall, 17 studies were included in the pairwise meta-analysis. Compared with BNT162b2, mRNA-1273 was associated with significantly reduced risk of SARS-CoV-2 infection (RR, 0.85 [95% CI, 0.75-0.97]; P=0.0151; I2 = 67.7%), severe SARS-CoV-2 infection (RR, 0.85 [95% CI, 0.77-0.93]; P=0.0009; I2 = 0%), COVID-19-associated hospitalization (RR, 0.88 [95% CI, 0.79-0.97]; P<0.0001; I2 = 0%), and COVID-19-associated mortality (RR, 0.63 [95% CI, 0.44-0.90]; P=0.0119; I2 = 0%) in IC populations. Results were consistent across subgroups. Because of sample size limitations, relative effectiveness of COVID-19 mRNA vaccines in IC populations cannot be studied in randomized trials. Based on nonrandomized studies, evidence certainty among comparisons was type 3 (low) and 4 (very low), reflecting potential biases in observational studies.
Conclusion: This GRADE meta-analysis based on a large number of consistent observational studies showed that the mRNA-1273 COVID-19 vaccine is associated with improved clinical effectiveness in IC populations compared with BNT162b2.
Keywords: BNT162b2; COVID-19; SARS-CoV-2; effectiveness; immunocompromised; mRNA vaccine; mRNA-1273; severe acute respiratory syndrome coronavirus 2.
Copyright © 2023 Wang, Haeussler, Spellman, Phillips, Ramiller, Bausch-Jurken, Sharma, Krivelyova, Vats and Van de Velde.
Conflict of interest statement
XW, KH, PS, AK, and SV are employees of ICON plc, a clinical research organization paid by Moderna, Inc., to conduct the study. AS is an independent epidemiology consultant/director of Data Health Ltd, which provides health data consultancy services, and was paid by Moderna, Inc., to conduct aspects of this study. LP is an employee and owner of Data-Driven LLC and AR is a contractor of Data-Driven LLC, a research organization paid by Moderna, Inc. to conduct aspects of this study. MB-J and NV are employees of Moderna, Inc., and hold stock/stock options in the company. This study was funded by Moderna, Inc. Authors employed by Moderna, Inc. were involved in the study design, analysis and interpretation of data, the writing of the manuscript, and the decision to submit the manuscript for publication.
Figures
Similar articles
-
Comparative Effectiveness of mRNA-1273 and BNT162b2 COVID-19 Vaccines Among Older Adults: Systematic Literature Review and Meta-Analysis Using the GRADE Framework.Infect Dis Ther. 2024 Apr;13(4):779-811. doi: 10.1007/s40121-024-00936-z. Epub 2024 Mar 18. Infect Dis Ther. 2024. PMID: 38498109 Free PMC article.
-
Effectiveness of BNT162b2 and CoronaVac COVID-19 vaccination against asymptomatic and symptomatic infection of SARS-CoV-2 omicron BA.2 in Hong Kong: a prospective cohort study.Lancet Infect Dis. 2023 Apr;23(4):421-434. doi: 10.1016/S1473-3099(22)00732-0. Epub 2022 Dec 12. Lancet Infect Dis. 2023. PMID: 36521506 Free PMC article.
-
Real-world comparative effectiveness of mRNA-1273 and BNT162b2 vaccines among immunocompromised adults identified in administrative claims data in the United States.Vaccine. 2022 Nov 8;40(47):6730-6739. doi: 10.1016/j.vaccine.2022.09.025. Epub 2022 Sep 24. Vaccine. 2022. PMID: 36163093 Free PMC article.
-
Safety and effectiveness of vaccines against COVID-19 in children aged 5-11 years: a systematic review and meta-analysis.Lancet Child Adolesc Health. 2023 Jun;7(6):379-391. doi: 10.1016/S2352-4642(23)00078-0. Epub 2023 Apr 18. Lancet Child Adolesc Health. 2023. PMID: 37084750 Free PMC article.
-
The effectiveness of BNT162b2 mRNA vaccine against COVID-19 caused by Delta variant of SARS-CoV-2: a systematic review and meta-analysis.Inflammopharmacology. 2022 Feb;30(1):149-157. doi: 10.1007/s10787-021-00915-7. Epub 2022 Jan 31. Inflammopharmacology. 2022. PMID: 35099680 Free PMC article.
Cited by
-
Delphi Panel Consensus Statement Generation: COVID-19 Vaccination Recommendations for Immunocompromised Populations in the European Union.Infect Dis Ther. 2024 Nov;13(11):2227-2253. doi: 10.1007/s40121-024-01051-9. Epub 2024 Oct 9. Infect Dis Ther. 2024. PMID: 39382830 Free PMC article.
-
Comparative Effectiveness of mRNA-1273 and BNT162b2 COVID-19 Vaccines Among Older Adults: Systematic Literature Review and Meta-Analysis Using the GRADE Framework.Infect Dis Ther. 2024 Apr;13(4):779-811. doi: 10.1007/s40121-024-00936-z. Epub 2024 Mar 18. Infect Dis Ther. 2024. PMID: 38498109 Free PMC article.
-
Comparative Effectiveness of mRNA-1273 and BNT162b2 COVID-19 Vaccines Among Adults with Underlying Medical Conditions: Systematic Literature Review and Pairwise Meta-Analysis Using GRADE.Adv Ther. 2025 May;42(5):2040-2077. doi: 10.1007/s12325-025-03117-7. Epub 2025 Mar 10. Adv Ther. 2025. PMID: 40063213 Free PMC article.
-
Reactogenicity Differences between Adjuvanted, Protein-Based and Messenger Ribonucleic Acid (mRNA)-Based COVID-19 Vaccines.Vaccines (Basel). 2024 Jul 19;12(7):802. doi: 10.3390/vaccines12070802. Vaccines (Basel). 2024. PMID: 39066440 Free PMC article.
-
Retrospective, Observational Analysis on the Impact of SARS-CoV-2 Variant Omicron in Hospitalized Immunocompromised Patients in a German Hospital Network-The VISAGE Study.Vaccines (Basel). 2024 Jun 7;12(6):634. doi: 10.3390/vaccines12060634. Vaccines (Basel). 2024. PMID: 38932363 Free PMC article.
References
-
- National Center for Immunization and Respiratory Diseases (NCIRD) Division of Viral Diseases . COVID data tracker weekly review: interpretive summary for February 10, 2023 (2023). Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html (Accessed April 4, 2023).
-
- SPIKEVAX (mRNA-1273) . Full prescribing information. Cambridge, MA: ModernaTX, Inc. (2022).
-
- COMIRNATY (BNT162b2) . Full prescribing information. New York, NY: Pfizer/BioNTech; (2022).
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

