Nasopharyngeal Carcinoma: Case Presentation and Literature Review of Treatment Innovation with Immunotherapy
- PMID: 37771655
- PMCID: PMC10547533
- DOI: 10.1055/s-0043-1774333
Nasopharyngeal Carcinoma: Case Presentation and Literature Review of Treatment Innovation with Immunotherapy
Abstract
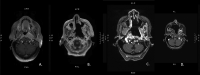
Nasopharyngeal carcinoma (NPC), a rare head and neck malignancy, arises from the epithelial lining of nasopharyngeal mucosa. The confluence of various risk factors, such as latent Epstein-Barr virus infection, genetic susceptibility, smoking, alcohol consumption, and high nitrosamine diet, is thought to contribute to NPC pathogenesis. Radiation therapy serves as the mainstay of treatment for early stage while concurrent chemotherapy and radiation are the basis of treatment for locoregional advanced disease with overall 80% five-year survival rate. Recurrent or metastatic disease pose treatment challenges as reirradiation, repeat cycles of chemotherapy, and surgery follow with high likelihood of treatment toxicity or postoperative morbidities. Typically reserved for nonresectable recurrent or metastatic disease, immunotherapy serves as novel treatment for NPC. NPC tumor microenvironment predominated by a dense infiltrate of immune cells hosts an ideal target for immunotherapy. Several clinical trials have investigated the efficacy of anti-programmed cell death protein 1 antibodies such as pembrolizumab, nivolumab, and camrelizumab with promising results. Treatment of recurrent and metastatic NPC remains a challenge; however, the advent of immunotherapy has provided additional options and potential for preventative and therapeutic measures.
Keywords: EBV; adoptive cell therapy; head and neck cancer; immune checkpoint inhibitors; immunotherapy; nasopharyngeal carcinoma; nasopharynx; recurrent disease.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. ( https://creativecommons.org/licenses/by-nc-nd/4.0/ ).
Conflict of interest statement
Conflict of Interest None declared.
Figures

References
-
- Chen Y P, Chan A TC, Le Q T, Blanchard P, Sun Y, Ma J.Nasopharyngeal carcinoma Lancet 2019394(10192):64–80. - PubMed
-
- Chen L, Hu C S, Chen X Z et al. Adjuvant chemotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase 3 multicentre randomised controlled trial. Eur J Cancer. 2017;75:150–158. - PubMed
-
- Kong L, Zhang Y, Hu C, Guo Y, Lu J J. Effects of induction docetaxel, platinum, and fluorouracil chemotherapy in patients with stage III or IVA/B nasopharyngeal cancer treated with concurrent chemoradiation therapy: final results of 2 parallel phase 2 clinical trials. Cancer. 2017;123(12):2258–2267. - PubMed
-
- Li W-F, Chen N-Y, Zhang N et al. Concurrent chemoradiotherapy with/without induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: long-term results of phase 3 randomized controlled trial. Int J Cancer. 2019;145(01):295–305. - PubMed
-
- Yang Q, Cao S-M, Guo L et al. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase III multicentre randomised controlled trial. Eur J Cancer. 2019;119:87–96. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

