Automated advanced imaging in acute ischemic stroke. Certainties and uncertainties
- PMID: 37771657
- PMCID: PMC10523426
- DOI: 10.1016/j.ejro.2023.100524
Automated advanced imaging in acute ischemic stroke. Certainties and uncertainties
Abstract
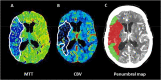
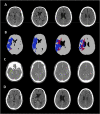
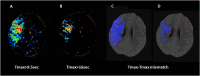
The purpose of this is study was to review pearls and pitfalls of advanced imaging, such as computed tomography perfusion and diffusion-weighed imaging and perfusion-weighted imaging in the selection of acute ischemic stroke (AIS) patients suitable for endovascular treatment (EVT) in the late time window (6-24 h from symptom onset). Advanced imaging can quantify infarct core and ischemic penumbra using specific threshold values and provides optimal selection parameters, collectively called target mismatch. More precisely, target mismatch criteria consist of core volume and/or penumbra volume and mismatch ratio (the ratio between total hypoperfusion and core volumes) with precise cut-off values. The parameters of target mismatch are automatically calculated with dedicated software packages that allow a quick and standardized interpretation of advanced imaging. However, this approach has several limitations leading to a misclassification of core and penumbra volumes. In fact, automatic software platforms are affected by technical artifacts and are not interchangeable due to a remarkable vendor-dependent variability, resulting in different estimate of target mismatch parameters. In addition, advanced imaging is not completely accurate in detecting infarct core, that can be under- or overestimated. Finally, the selection of candidates for EVT remains currently suboptimal due to the high rates of futile reperfusion and overselection caused by the use of very stringent inclusion criteria. For these reasons, some investigators recently proposed to replace advanced with conventional imaging in the selection for EVT, after the demonstration that non-contrast CT ASPECTS and computed tomography angiography collateral evaluation are not inferior to advanced images in predicting outcome in AIS patients treated with EVT. However, other authors confirmed that CTP and PWI/DWI postprocessed images are superior to conventional imaging in establishing the eligibility of patients for EVT. Therefore, the routine application of automatic assessment of advanced imaging remains a matter of debate. Recent findings suggest that the combination of conventional and advanced imaging might improving our selection criteria.
Keywords: Advanced imaging; Automatic software (maximum 6); Patient selection; Reperfusion therapies; Stroke.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Higher agreement in endovascular treatment decision-making than in parametric quantifications among automated CT perfusion software packages in acute ischemic stroke.J Xray Sci Technol. 2021;29(5):823-834. doi: 10.3233/XST-210898. J Xray Sci Technol. 2021. PMID: 34334443
-
Penumbra quantification from MR SWI-DWI mismatch and its comparison with MR ASL PWI-DWI mismatch in patients with acute ischemic stroke.NMR Biomed. 2021 Jul;34(7):e4526. doi: 10.1002/nbm.4526. Epub 2021 Apr 20. NMR Biomed. 2021. PMID: 33880799
-
Automated prediction of final infarct volume in patients with large-vessel occlusion acute ischemic stroke.Neurosurg Focus. 2021 Jul;51(1):E13. doi: 10.3171/2021.4.FOCUS21134. Neurosurg Focus. 2021. PMID: 34198252 Free PMC article.
-
Multimodal CT in Acute Stroke.Curr Neurol Neurosci Rep. 2019 Jul 27;19(9):63. doi: 10.1007/s11910-019-0978-z. Curr Neurol Neurosci Rep. 2019. PMID: 31352585 Review.
-
[Intravenous administration of a tissue plasminogen activator beyond 3 hours of the onset of acute ischemic stroke--MRI-based decision making].Brain Nerve. 2008 Oct;60(10):1173-80. Brain Nerve. 2008. PMID: 18975605 Review. Japanese.
Cited by
-
Target mismatch criteria in acute ischemic stroke patients with distal-medium vessel occlusion.Eur Stroke J. 2025 Aug 11:23969873251362205. doi: 10.1177/23969873251362205. Online ahead of print. Eur Stroke J. 2025. PMID: 40790832 Free PMC article.
-
Quantitative Evaluation of Ischemic Core Volume in GE's CT Perfusion Imaging Analysis Software and Its Relationship to Alberta Stroke Program Early CT Score.J Neuroendovasc Ther. 2025;19(1):2024-0090. doi: 10.5797/jnet.oa.2024-0090. Epub 2025 Feb 27. J Neuroendovasc Ther. 2025. PMID: 40034101 Free PMC article.
-
Current Stroke Solutions Using Artificial Intelligence: A Review of the Literature.Brain Sci. 2024 Nov 26;14(12):1182. doi: 10.3390/brainsci14121182. Brain Sci. 2024. PMID: 39766381 Free PMC article. Review.
References
-
- Campbell B.C.V., Khatri P. Stroke. Lancet. 2020;396:129–142. - PubMed
-
- Powers W.J. Acute ischemic stroke. N. Engl. J. Med. 2020;383:252–260. - PubMed
-
- Ermine C.M., Bivard A., Parsons M.W., Baron J.C. The ischemic penumbra: from concept to reality. Int. J. Stroke. 2021;16:497–509. - PubMed
-
- Baron J.C. Protecting the ischaemic penumbra as an adjunct to thrombectomy for acute stroke. Nat. Rev. Neurol. 2018;14:325–337. - PubMed
-
- Heiss W.-D., Zaro-Weber O. Extension of therapeutic window in ischemic stroke by selective mismatch imaging. Int. J. Stroke. 2019;14:351–358. - PubMed
LinkOut - more resources
Full Text Sources

