Targeting cullin neddylation for cancer and fibrotic diseases
- PMID: 37771770
- PMCID: PMC10526667
- DOI: 10.7150/thno.78876
Targeting cullin neddylation for cancer and fibrotic diseases
Abstract
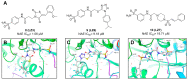
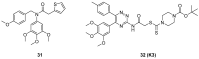
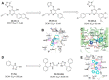
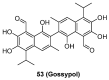
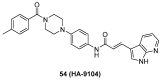
Protein neddylation is a post-translational modification, and its best recognized substrates are cullin family proteins, which are the core component of Cullin-RING ligases (CRLs). Given that most neddylation pathway proteins are overactivated in different cancers and fibrotic diseases, targeting neddylation becomes an emerging approach for the treatment of these diseases. To date, numerous neddylation inhibitors have been developed, of which MLN4924 has entered phase I/II/III clinical trials for cancer treatment, such as acute myeloid leukemia, melanoma, lymphoma and solid tumors. Here, we systematically describe the structures and biological functions of the critical enzymes in neddylation, highlight the medicinal chemistry advances in the development of neddylation inhibitors and propose the perspectives concerning targeting neddylation for cancer and fibrotic diseases.
Keywords: Cancer; Cullin neddylation; Fibrotic diseases; Inhibitors.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures











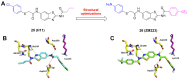

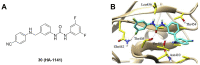



References
-
- Soucy TA, Smith PG, Rolfe M. Targeting NEDD8-activated cullin-ring ligases for the treatment of cancer. Clin Cancer Res. 2009;15:3912–6. - PubMed
-
- Watson IR, Irwin MS, Ohh M. NEDD8 pathways in cancer, sine quibus non. Cancer Cell. 2011;19:168–76. - PubMed
-
- Mendoza HM, Shen LN, Botting C, Lewis A, Chen J, Ink B. et al. NEDP1, a highly conserved cysteine protease that deneddylates cullins. J Biol Chem. 2003;278:25637–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

