Tailoring the pore size of expanded porphyrinoids for lanthanide selectivity
- PMID: 37771918
- PMCID: PMC10523133
- DOI: 10.1039/d3ra05710k
Tailoring the pore size of expanded porphyrinoids for lanthanide selectivity
Abstract
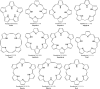
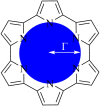
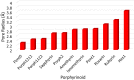
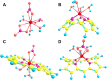
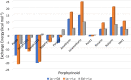
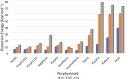
Despite increase in demand, capacity for the recycling of rare earth elements remains limited, partly due to the inefficiencies with processes currently utilised in the separation of lanthanides. This study highlights the potential use of expanded porphyrinoids in lanthanide separation through selective binding, dependent on the tailored pore size of the macrocycle. Each emerging trend is subjected to multi-factored analysis to decompose the underlying source. Results promote the viability of size-based separation with preferential binding of larger lanthanum(iii) ions to amethyrin and isoamethyrin macrocycles, while smaller macrocycles such as pentaphyrin(0.0.0.0.0) present a preferential binding of lutetium(iii) ions. Additionally, the porphyrin(2.2.2.2) macrocycle shows a selectivity for gadolinium(iii) ions over both larger and smaller ions. An upper limit of applicable pore size is shown to be ≈2.8 Å, beyond which the formed complexes are predicted to be less stable than the corresponding nitrate complexes.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
LinkOut - more resources
Full Text Sources

