Naphthopyran molecular switches and their emergent mechanochemical reactivity
- PMID: 37772118
- PMCID: PMC10530568
- DOI: 10.1039/d3sc03729k
Naphthopyran molecular switches and their emergent mechanochemical reactivity
Abstract
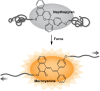
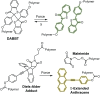
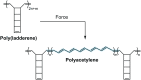
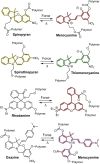
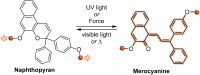
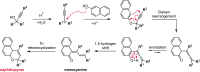
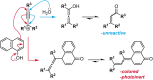
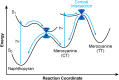
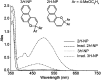
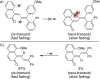
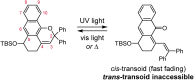
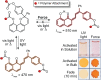
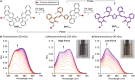
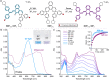
Naphthopyran molecular switches undergo a ring-opening reaction upon external stimulation to generate intensely colored merocyanine dyes. Their unique modularity and synthetic accessibility afford exceptional control over their properties and stimuli-responsive behavior. Commercial applications of naphthopyrans as photoswitches in photochromic ophthalmic lenses have spurred an extensive body of work exploring naphthopyran-merocyanine structure-property relationships. The recently discovered mechanochromic behavior of naphthopyrans has led to their emergent application in the field of polymer mechanochemistry, enabling advances in the design of force-responsive materials as well as fundamental insights into mechanochemical reactivity. The structure-property relationships established in the photochemical literature serve as a convenient blueprint for the design of naphthopyran molecular force probes with precisely tuned properties. On the other hand, the mechanochemical reactivity of naphthopyran diverges in many cases from the conventional photochemical pathways, resulting in unexpected properties and opportunities for deeper understanding and innovation in polymer mechanochemistry. Here, we highlight the features of the naphthopyran scaffold that render it a powerful platform for the design of mechanochromic materials and review recent advances in naphthopyran mechanochemistry.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

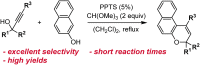











References
-
- Berkowski K. L. Potisek S. L. Hickenboth C. R. Moore J. S. Macromolecules. 2005;38:8975–8978. doi: 10.1021/ma051394n. - DOI
Publication types
LinkOut - more resources
Full Text Sources

