Dimeric tetrabromo- p-quinodimethanes: synthesis and structural/electronic properties
- PMID: 37772123
- PMCID: PMC10530488
- DOI: 10.1039/d3sc01615c
Dimeric tetrabromo- p-quinodimethanes: synthesis and structural/electronic properties
Abstract
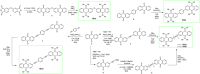
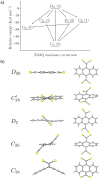
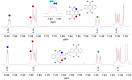
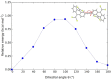
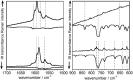
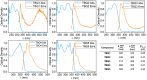
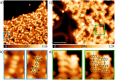
Despite their great potential as molecular building blocks for organic synthesis, tetrabromo-p-quinodimethanes (TBQs) are a relatively unknown family of compounds. Herein, we showcase a series of five derivatives incorporating two tetrabromo-anthraquinodimethane (TBAQ) units linked by π-conjugated spacers of different nature and length. The resulting dimers TBQ1-5 are fully characterised by means of thorough spectroscopic measurements and theoretical calculations. Interestingly, owing to the steric hindrance imposed by the four bulky bromine atoms, the TBAQ fragments adopt a characteristically warped geometry, somehow resemblant of a butterfly, and the novel dimers show a complex NMR pattern with signal splittings. To ascertain whether dynamic processes regarding fluxional inversion of the butterfly configurations are involved, first-principles calculations assessing the interconversion energy barriers are performed. Three possible stereoisomers are predicted involving two diastereomers, thus accounting for the observed NMR spectra. The rotational freedom of the TBAQ units around the π-conjugated linker influences the structural and electronic properties of TBQ1-5 and modulates the electronic communication between the terminal TBAQ moieties. The role of the linker on the electronic properties is investigated by Raman and UV-vis spectroscopies, theoretical calculations and UV-vis measurements at low temperature. TBQ1-5 are of interest as less-explored structural building precursors for a variety of scientific areas. Finally, the sublimation, self-assembly and reactivity on Au(111) of TBQ3 is assessed.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures


References
-
- Desai N. B. McKelvie N. Ramirez F. J. Am. Chem. Soc. 1962;84:1745–1747. doi: 10.1021/ja00868a057. - DOI
-
- Corey E. J. Fuchs P. L. Tetrahedron Lett. 1972;13:3769–3772. doi: 10.1016/S0040-4039(01)94157-7. - DOI
-
- Iyoda M. Tanaka S. Otani H. Nose M. Oda M. J. Am. Chem. Soc. 1988;110:8494–8500. doi: 10.1021/ja00233a028. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

