Unraveling the Most Relevant Features for the Design of Iridium Mixed Oxides with High Activity and Durability for the Oxygen Evolution Reaction in Acidic Media
- PMID: 37772191
- PMCID: PMC10523372
- DOI: 10.1021/jacsau.3c00247
Unraveling the Most Relevant Features for the Design of Iridium Mixed Oxides with High Activity and Durability for the Oxygen Evolution Reaction in Acidic Media
Abstract
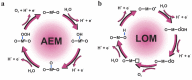
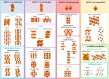
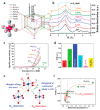
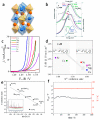
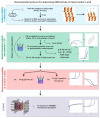
Proton exchange membrane water electrolysis (PEMWE) is the technology of choice for the large-scale production of green hydrogen from renewable energy. Current PEMWEs utilize large amounts of critical raw materials such as iridium and platinum in the anode and cathode electrodes, respectively. In addition to its high cost, the use of Ir-based catalysts may represent a critical bottleneck for the large-scale production of PEM electrolyzers since iridium is a very expensive, scarce, and ill-distributed element. Replacing iridium from PEM anodes is a challenging matter since Ir-oxides are the only materials with sufficient stability under the highly oxidant environment of the anode reaction. One of the current strategies aiming to reduce Ir content is the design of advanced Ir-mixed oxides, in which the introduction of cations in different crystallographic sites can help to engineer the Ir active sites with certain characteristics, that is, environment, coordination, distances, oxidation state, etc. This strategy comes with its own problems, since most mixed oxides lack stability during the OER in acidic electrolyte, suffering severe structural reconstruction, which may lead to surfaces with catalytic activity and durability different from that of the original mixed oxide. Only after understanding such a reconstruction process would it be possible to design durable and stable Ir-based catalysts for the OER. In this Perspective, we highlight the most successful strategies to design Ir mixed oxides for the OER in acidic electrolyte and discuss the most promising lines of evolution in the field.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Fuel Cells and Hydrogen Joint Undertaking (FCH) . Hydrogen Roadmap Europe - a Sustainable Pathway for the European Energy Transition; Publications Office of the European Union, 2019.10.2843/249013. - DOI
-
- United States Department of State . Long-Term Strategy of the United States: Pathways to Net-Zero Greenhouse Gas Emissions by 2050; United States Department of State and the United States Executive Office of the President: Washington, DC, 2021. https://www.whitehouse.gov/wp-content/uploads/2021/10/US-Long-Term-Strat....
-
- Zhao A.; Kennedy S.; Keefe K. O.; Borreo M.; Clark-sutton K.; Cui R.; Dahl C.; Deye G.; Feldmann J.; Kennedy K.; Mcjeon H.; Moravec M.; Nilov D.; Rajpurohit S.; Rosas J.; Squire C.; Hultman N.. An All-In Pathway To 2030: The Beyond 50 Scenario; 2022. https://americaisallin.com/sites/default/files/2022-11/Final%20-%20The%2....
-
- Internationan Energy Agency (IEA) . Renewables 2022; IEA: Paris, 2022. https://www.iea.org/reports/renewables-2022.
-
- Chatenet M.; Pollet B. G.; Dekel D. R.; Dionigi F.; Deseure J.; Millet P.; Braatz R. D.; Bazant M. Z.; Eikerling M.; Staffell I.; Balcombe P.; Shao-Horn Y.; Schäfer H. Water Electrolysis: From Textbook Knowledge to the Latest Scientific Strategies and Industrial Developments. Chem. Soc. Rev. 2022, 51 (11), 4583–4762. 10.1039/D0CS01079K. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
