Antibacterial activity and antibacterial mechanism of flavaspidic acid BB against Staphylococcus haemelyticus
- PMID: 37773054
- PMCID: PMC10540430
- DOI: 10.1186/s12866-023-02997-5
Antibacterial activity and antibacterial mechanism of flavaspidic acid BB against Staphylococcus haemelyticus
Abstract
Background: Staphylococcus haemolyticus (S. haemolyticus) is the main etiological factor in skin and soft tissue infections (SSTI). S. haemolyticus infections are an important concern worldwide, especially with the associated biofilms and drug resistance. Herein, we investigated the inhibitory effect of Flavaspidic acid BB obtained from plant extractions on clinical S. haemolyticus strains and their biofilms. Moreover, we predicted its ability to bind to the protein-binding site by molecular simulation. Since the combination of Hsp70 and RNase P synthase after molecular simulation with flavaspidic acid BB is relatively stable, enzyme-linked immunosorbent assay (ELISA) was used to investigate Hsp70 and RNase P synthase to verify the potential antimicrobial targets of flavaspidic acid BB.
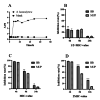
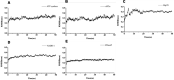
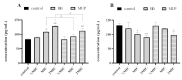
Results: The minimum inhibitory concentrations (MIC) of flavaspidic acid BB on 16 clinical strains of S. haemolyticus was 5 ~ 480 µg/mL, and BB had a slightly higher inhibitory effect on the biofilm than MUP. The inhibitory effect of flavaspidic acid BB on biofilm formation was better with an increase in the concentration of BB. Molecular simulation verified its ability to bind to the protein-binding site. The combination of ELISA kits showed that flavaspidic acid BB promoted the activity of Hsp70 and inhibited the activity of RNase P, revealing that flavaspidic acid BB could effectively inhibit the utilization and re-synthesis of protein and tRNA synthesis, thus inhibiting bacterial growth and biofilm formation to a certain extent.
Conclusions: This study could potentially provide a new prospect for the development of flavaspidic acid BB as an antibacterial agent for resistant strains.
Keywords: Anti-bacterial activity; Anti-biofilm activity; Dryopteris fragrans (L.) Schott; Flavaspidic acid BB; Molecular docking; Staphylococcus haemolyticus.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



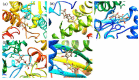
Similar articles
-
Flavaspidic acid BB combined with mupirocin improves its anti-bacterial and anti-biofilm activities against Staphylococcus epidermidis.BMC Microbiol. 2022 Jul 15;22(1):179. doi: 10.1186/s12866-022-02578-y. BMC Microbiol. 2022. PMID: 35840879 Free PMC article.
-
Evaluation of the antimicrobial function of Ginkgo biloba exocarp extract against clinical bacteria and its effect on Staphylococcus haemolyticus by disrupting biofilms.J Ethnopharmacol. 2022 Nov 15;298:115602. doi: 10.1016/j.jep.2022.115602. Epub 2022 Aug 24. J Ethnopharmacol. 2022. PMID: 36030030
-
Antibacterial and anti-biofilm activities of Disaspidin BB against Staphylococcus epidermidis.Front Microbiol. 2023 Jan 19;14:999449. doi: 10.3389/fmicb.2023.999449. eCollection 2023. Front Microbiol. 2023. PMID: 36744091 Free PMC article.
-
Antibacterial activity of two phloroglucinols, flavaspidic acids AB and PB, from Dryopteris crassirhizoma.Arch Pharm Res. 2009 May;32(5):655-9. doi: 10.1007/s12272-009-1502-9. Epub 2009 May 27. Arch Pharm Res. 2009. PMID: 19471878 Free PMC article.
-
Antibacterial and antibiofilm efficacy of Solanum lasiocarpum root extract synthesized silver/silver chloride nanoparticles against Staphylococcus haemolyticus associated with bovine mastitis.Microb Pathog. 2024 Jul;192:106724. doi: 10.1016/j.micpath.2024.106724. Epub 2024 Jun 2. Microb Pathog. 2024. PMID: 38834135
Cited by
-
Sulforaphane-Enriched Extracts from Broccoli Exhibit Antimicrobial Activity against Plant Pathogens, Promising a Natural Antimicrobial Agent for Crop Protection.Biomolecules. 2024 Mar 14;14(3):352. doi: 10.3390/biom14030352. Biomolecules. 2024. PMID: 38540770 Free PMC article.
References
-
- Takeuchi F, Watanabe S, Baba T, Yuzawa H, Ito T, Morimoto Y, Kuroda M, Cui LZ, Takahashi M, Ankai A, Baba S, Fukui S, Lee JC, Hiramatsu K. Whole-genome sequencing of staphylococcus haemolyticus uncovers the extreme plasticity of its genome and the evolution of human-colonizing staphylococcal species. J Bacteriol. 2005;187(21):7292–308. doi: 10.1128/JB.187.21.7292-7308.2005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

