High-Dose Inhaled Nitric Oxide in Acute Hypoxemic Respiratory Failure Due to COVID-19: A Multicenter Phase II Trial
- PMID: 37774011
- PMCID: PMC10765403
- DOI: 10.1164/rccm.202304-0637OC
High-Dose Inhaled Nitric Oxide in Acute Hypoxemic Respiratory Failure Due to COVID-19: A Multicenter Phase II Trial
Abstract
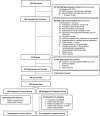
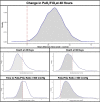
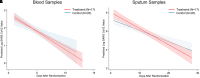
Rationale: The effects of high-dose inhaled nitric oxide on hypoxemia in coronavirus disease (COVID-19) acute respiratory failure are unknown. Objectives: The primary outcome was the change in arterial oxygenation (PaO2/FiO2) at 48 hours. The secondary outcomes included: time to reach a PaO2/FiO2.300mmHg for at least 24 hours, the proportion of participants with a PaO2/FiO2.300mmHg at 28 days, and survival at 28 and at 90 days. Methods: Mechanically ventilated adults with COVID-19 pneumonia were enrolled in a phase II, multicenter, single-blind, randomized controlled parallel-arm trial. Participants in the intervention arm received inhaled nitric oxide at 80 ppm for 48 hours, compared with the control group receiving usual care (without placebo). Measurements and Main Results: A total of 193 participants were included in the modified intention-to-treat analysis. The mean change in PaO2/FiO2 ratio at 48 hours was 28.3mmHg in the intervention group and 21.4mmHg in the control group (mean difference, 39.1mmHg; 95% credible interval [CrI], 18.1 to 60.3). The mean time to reach a PaO2/FiO2.300mmHg in the interventional group was 8.7 days, compared with 8.4 days for the control group (mean difference, 0.44; 95% CrI, 23.63 to 4.53). At 28 days, the proportion of participants attaining a PaO2/FiO2.300mmHg was 27.7% in the inhaled nitric oxide group and 17.2% in the control subjects (risk ratio, 2.03; 95% CrI, 1.11 to 3.86). Duration of ventilation and mortality at 28 and 90 days did not differ. No serious adverse events were reported. Conclusions: The use of high-dose inhaled nitric oxide resulted in an improvement of PaO2/FiO2 at 48 hours compared with usual care in adults with acute hypoxemic respiratory failure due to COVID-19.
Keywords: COVID-19; critical illness; nitric oxide; pneumonia; respiration, artificial; viremia.
Figures

Comment in
-
Inhaled NO in COVID-19 Acute Respiratory Distress Syndrome: Yes or No?Am J Respir Crit Care Med. 2023 Dec 15;208(12):1259-1261. doi: 10.1164/rccm.202310-1823ED. Am J Respir Crit Care Med. 2023. PMID: 37934465 Free PMC article. No abstract available.
-
High-Dose Inhaled Nitric Oxide in Acute Hypoxemic Respiratory Failure: Need for Patient Phenotyping?Am J Respir Crit Care Med. 2024 Feb 15;209(4):459-460. doi: 10.1164/rccm.202310-1909LE. Am J Respir Crit Care Med. 2024. PMID: 38128106 Free PMC article. No abstract available.
References
-
- Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/nda/99/20845_INOmax.cfm
-
- Frostell C, Fratacci MD, Wain JC, Jones R, Zapol WM. Inhaled nitric oxide: a selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation . 1991;83:2038–2047. - PubMed
-
- Roberts JD, Polaner DM, Lang P, Zapol WM. Inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Lancet . 1992;340:818–819. - PubMed
-
- European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/inomax#overview-section
-
- Japan Pharmaceuticals and Medical Devices Agency. 2015. https://www.pmda.go.jp/files/000229077.pdf
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

