Cold exposure induces vaso-occlusion and pain in sickle mice that depend on complement activation
- PMID: 37774369
- PMCID: PMC10731576
- DOI: 10.1182/blood.2022019282
Cold exposure induces vaso-occlusion and pain in sickle mice that depend on complement activation
Abstract
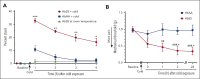
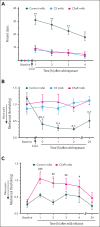
Vaso-occlusive pain episodes (VOE) cause severe pain in patients with sickle cell disease (SCD). Vaso-occlusive events promote ischemia/reperfusion pathobiology that activates complement. We hypothesized that complement activation is linked to VOE. We used cold to induce VOE in the Townes sickle homozygous for hemoglobin S (HbSS) mouse model and complement inhibitors to determine whether anaphylatoxin C5a mediates VOE. We used a dorsal skinfold chamber to measure microvascular stasis (vaso-occlusion) and von Frey filaments applied to the plantar surface of the hind paw to assess mechanical hyperalgesia in HbSS and control Townes mice homozygous for hemoglobin A (HbAA) mice after cold exposure at 10°C/50°F for 1 hour. Cold exposure induced more vaso-occlusion in nonhyperalgesic HbSS mice (33%) than in HbAA mice (11%) or HbSS mice left at room temperature (1%). Cold exposure also produced mechanical hyperalgesia as measured by paw withdrawal threshold in HbSS mice compared with that in HbAA mice or HbSS mice left at room temperature. Vaso-occlusion and hyperalgesia were associated with an increase in complement activation fragments Bb and C5a in plasma of HbSS mice after cold exposure. This was accompanied by an increase in proinflammatory NF-κB activation and VCAM-1 and ICAM-1 expression in the liver. Pretreatment of nonhyperalgesic HbSS mice before cold exposure with anti-C5 or anti-C5aR monoclonal antibodies (mAbs) decreased vaso-occlusion, mechanical hyperalgesia, complement activation, and liver inflammatory markers compared with pretreatment with control mAb. Anti-C5 or -C5aR mAb infusion also abrogated mechanical hyperalgesia in HbSS mice with ongoing hyperalgesia at baseline. These findings suggest that C5a promotes vaso-occlusion, pain, and inflammation during VOE and may play a role in chronic pain.
© 2023 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: J. D. Belcher, G.M.V., and D.A.S. have research funding from Omeros. J. D. Belcher and G.M.V. have research funding from CSL Behring, Hillhurst Biopharmaceuticals, Sanofi, and Astellas/Mitobridge; and both are consultants for Sanofi and Astellas/Mitobridge. G.M.V. also is on data safety monitoring committees for Alexion and Novo Nordisk trials. K.G. reports honoraria from Novartis and CSL Behring and research grants from Cyclerion, 1910 Genetics, Novartis, Grifols, Zilker, UCI Foundation, and SCIRE Foundation. J. D. Beckman has research funding from Bayer. The remaining authors declare no competing financial interests.
Figures




Comment in
-
Cold comfort in sickle cell disease.Blood. 2023 Nov 30;142(22):1854-1856. doi: 10.1182/blood.2023022621. Blood. 2023. PMID: 38032675 No abstract available.
References
-
- Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991;325(1):11–16. - PubMed
-
- Sagi V, Song-Naba WL, Benson BA, Joshi SS, Gupta K. Mouse models of pain in sickle cell disease. Curr Protoc Neurosci. 2018;85(1):e54. - PubMed
-
- Kalambur VS, Mahaseth H, Bischof JC, et al. Microvascular blood flow and stasis in transgenic sickle mice: utility of a dorsal skin fold chamber for intravital microscopy. Am J Hematol. 2004;77(2):117–125. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

