Anti-EGFR Antibodies in the Management of Advanced Colorectal Cancer
- PMID: 37774394
- PMCID: PMC11025386
- DOI: 10.1093/oncolo/oyad262
Anti-EGFR Antibodies in the Management of Advanced Colorectal Cancer
Abstract
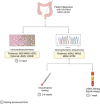
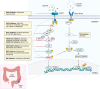
Colorectal cancer is the third most common cancer worldwide, and incidence is rising in younger individuals. Anti-EGFR antibodies, including cetuximab and panitumumab, have been incorporated into standard-of-care practice for patients with advanced disease. Herein, we review the molecular characteristics of these agents and the trials that lead to their approvals. Further, we discuss clinical implications of data regarding biomarkers that dictate treatment selection, different dosing strategies, and side effect management. Finally, we look towards the future and describe contexts in which these agents are currently being investigated clinically with a focus on combinations with MAPK-targeted therapies and immunotherapy. Overall, this review provides historical context, current clinical usage, and future directions for anti-EGFR antibodies in advanced colorectal cancer.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
Pashtoon Murtaza Kasi owns stock in Elicio Therapeutics; has consulted or advised for Natera, Foundation Medicine, MSD Oncology, Tempus, Bayer, Eli Lilly, Delcath Systems, QED Therapeutics, Servier, Taiho Oncology, Exact Sciences, Daiichi Sankyo/AstraZeneca, Eisai, Seattle Genetics, SAGA Diagnostics, Illumina, BostonGene, NeoGenomics Laboratories, Elicio Therapeutics, and Ipsen; has received institutional research funding from Advanced Accelerator Applications, Tersera, and Boston Scientific; and has received reimbursement for travel expenses from AstraZeneca. Manuel Geroy Afable and Cameron Herting are employees of Eli Lilly and Company. Mariusz Lukanowski was an employee of Eli Lilly and Company, with ownership interests, until the finalization of this review article and left the organization at the time of submission. Zhaohui Jin has received institutional funding for scientific advising from QED Therapeutics, Novartis, Daiichi Sankyo/AstraZeneca, Eli Lilly, and GSK.
Figures


References
-
- Colorectal cancer statistics. World Cancer Research Fund International; 2020. Accessed June 6, 2022. https://www.wcrf.org/cancer-trends/colorectal-cancer-statistics/
-
- Colorectal cancer statistics. Centers for Disease Control and Prevention; 2021. Accessed June 6, 2022. https://www.cdc.gov/cancer/colorectal/statistics/index.htm
-
- Key statistics for colorectal cancer. American Cancer Society. Accessed June 6, 2022. https://www.cancer.org/cancer/colon-rectal-cancer/about/key-statistics.html
-
- Cancer stat facts: Colorectal cancer. National Cancer Institute; 2022. Accessed June 6, 2022. https://seer.cancer.gov/statfacts/html/colorect.html
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

