Upregulation of the NKG2D Ligand ULBP2 by JC Polyomavirus Infection Promotes Immune Recognition by Natural Killer Cells
- PMID: 37774496
- PMCID: PMC11175686
- DOI: 10.1093/infdis/jiad424
Upregulation of the NKG2D Ligand ULBP2 by JC Polyomavirus Infection Promotes Immune Recognition by Natural Killer Cells
Abstract
Background: JC polyomavirus (JCPyV) causes progressive multifocal leukoencephalopathy (PML), a potentially fatal complication of severe immune suppression with no effective treatment. Natural killer (NK) cells play critical roles in defense against viral infections; however, NK-cell response to JCPyV infection remains unexplored.
Methods: NK- and T-cell responses against the JCPyV VP1 were compared using intracellular cytokine staining upon stimulation with peptide pools. A novel flow cytometry-based assay was developed to determine NK-cell killing efficiency of JCPyV-infected astrocyte-derived SVG-A cells. Blocking antibodies were used to evaluate the contribution of NK-cell receptors in immune recognition of JCPyV-infected cells.
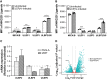
Results: In about 40% of healthy donors, we detected robust CD107a upregulation and IFN-γ production by NK cells, extending beyond T-cell responses. Next, using the NK-cell-mediated killing assay, we showed that coculture of NK cells and JCPyV-infected SVG-A cells leads to a 60% reduction in infection, on average. JCPyV-infected cells had enhanced expression of ULBP2-a ligand for the activating NK-cell receptor NKG2D, and addition of NKG2D blocking antibodies decreased NK-cell degranulation.
Conclusions: NKG2D-mediated activation of NK cells plays a key role in controlling JCPyV replication and may be a promising immunotherapeutic target to boost NK-cell anti-JCPyV activity.
Keywords: JC polyomavirus; NK cell antiviral immunity; NKG2D; ULBP.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



References
-
- Knowles WA, Pipkin P, Andrews N, et al. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol 2003; 71:115–23. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

