Phase II study of capecitabine-based concomitant chemoradiation followed by durvalumab as a neoadjuvant strategy in locally advanced rectal cancer: the PANDORA trial
- PMID: 37774508
- PMCID: PMC10594026
- DOI: 10.1016/j.esmoop.2023.101824
Phase II study of capecitabine-based concomitant chemoradiation followed by durvalumab as a neoadjuvant strategy in locally advanced rectal cancer: the PANDORA trial
Abstract
Background: This study investigated the efficacy of chemoradiotherapy (CRT) followed by durvalumab as neoadjuvant therapy of locally advanced rectal cancer.
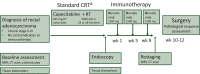
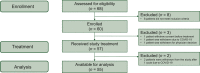
Patients and methods: The PANDORA trial is a prospective, phase II, open-label, single-arm, multicenter study aimed at evaluating the efficacy and safety of preoperative treatment with durvalumab (1500 mg every 4 weeks for three administrations) following long-course radiotherapy (RT) plus concomitant capecitabine (5040 cGy RT in 25-28 fractions over 5 weeks and capecitabine administered at 825 mg/m2 twice daily). The primary endpoint was the pathological complete response (pCR) rate; secondary endpoints were the proportion of clinical complete remissions and safety. The sample size was estimated assuming a null pCR proportion of 0.15 and an alternative pCR proportion of 0.30 (α = 0.05, power = 0.80). The proposed treatment could be considered promising if ≥13 pCRs were observed in 55 patients (EudraCT: 2018-004758-39; NCT04083365).
Results: Between November 2019 and August 2021, 60 patients were accrued, of which 55 were assessable for the study's objectives. Two patients experienced disease progression during treatment. Nineteen out of 55 eligible patients achieved a pCR (34.5%, 95% confidence interval 22.2% to 48.6%). Regarding toxicity related to durvalumab, grade 3 adverse events (AEs) occurred in four patients (7.3%) (diarrhea, skin toxicity, transaminase increase, lipase increase, and pancolitis). Grade 4 toxicity was not observed. In 20 patients (36.4%), grade 1-2 AEs related to durvalumab were observed. The most common were endocrine toxicity (hyper/hypothyroidism), dermatologic toxicity (skin rash), and gastrointestinal toxicity (transaminase increase, nausea, diarrhea, constipation).
Conclusion: This study met its primary endpoint showing that CRT followed by durvalumab could increase pCR with a safe toxicity profile. This combination is a promising, feasible strategy worthy of further investigation.
Keywords: durvalumab; immunotherapy; locally advanced rectal cancer; neoadjuvant strategy.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Bosset J.F., Collette L., Calais G., et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–1123. - PubMed
-
- Glynne-Jones R., Counsell N., Quirke P., et al. Chronicle: results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol. 2014;25(7):1356–1362. - PubMed
-
- Breugom A.J., van Gijn W., Muller E.W., et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann Oncol. 2015;26(4):696–701. - PubMed
-
- Hong Y.S., Kim S.Y., Lee J.S., et al. Oxaliplatin-based adjuvant chemotherapy for rectal cancer after preoperative chemoradiotherapy (ADORE): long-term results of a randomized controlled trial. J Clin Oncol. 2019;37(33):3111–3123. - PubMed
-
- Braendengen M., Tveit K.M., Berglund A., et al. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol. 2008;26(22):3687–3694. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous