Highly contractile 3D tissue engineered skeletal muscles from human iPSCs reveal similarities with primary myoblast-derived tissues
- PMID: 37774701
- PMCID: PMC10656354
- DOI: 10.1016/j.stemcr.2023.08.014
Highly contractile 3D tissue engineered skeletal muscles from human iPSCs reveal similarities with primary myoblast-derived tissues
Abstract
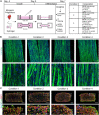
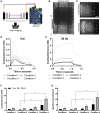
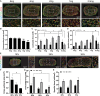
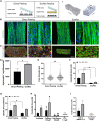
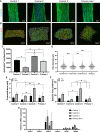
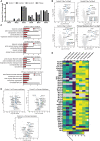
Skeletal muscle research is transitioning toward 3D tissue engineered in vitro models reproducing muscle's native architecture and supporting measurement of functionality. Human induced pluripotent stem cells (hiPSCs) offer high yields of cells for differentiation. It has been difficult to differentiate high-quality, pure 3D muscle tissues from hiPSCs that show contractile properties comparable to primary myoblast-derived tissues. Here, we present a transgene-free method for the generation of purified, expandable myogenic progenitors (MPs) from hiPSCs grown under feeder-free conditions. We defined a protocol with optimal hydrogel and medium conditions that allowed production of highly contractile 3D tissue engineered skeletal muscles with forces similar to primary myoblast-derived tissues. Gene expression and proteomic analysis between hiPSC-derived and primary myoblast-derived 3D tissues revealed a similar expression profile of proteins involved in myogenic differentiation and sarcomere function. The protocol should be generally applicable for the study of personalized human skeletal muscle tissue in health and disease.
Keywords: 3D-tissue engineering; contractile force; drug screening; induced pluripotent stem cells; myoblasts; myofiber; organ-on-a-chip; personalized medicine; satellite cell; skeletal muscle.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A.I., E.W., V.S., W.P., and J.G. are inventors on a patent in the field of muscle on a chip.
Figures

References
-
- Afshar M.E., Abraha H.Y., Bakooshli M.A., Davoudi S., Thavandiran N., Tung K., Ahn H., Ginsberg H.J., Zandstra P.W., Gilbert P.M. A 96-well culture platform enables longitudinal analyses of engineered human skeletal muscle microtissue strength. Sci. Rep. 2020;10:6918. doi: 10.1038/s41598-020-62837-8. - DOI - PMC - PubMed
-
- Afshar Bakooshli M., Lippmann E.S., Mulcahy B., Iyer N., Nguyen C.T., Tung K., Stewart B.A., van den Dorpel H., Fuehrmann T., Shoichet M., et al. A 3D culture model of innervated human skeletal muscle enables studies of the adult neuromuscular junction. Elife. 2019;8 doi: 10.7554/eLife.44530. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

