Antidepressant and antipsychotic side-effects and personalised prescribing: a systematic review and digital tool development
- PMID: 37774723
- PMCID: PMC10878984
- DOI: 10.1016/S2215-0366(23)00262-6
Antidepressant and antipsychotic side-effects and personalised prescribing: a systematic review and digital tool development
Abstract
Background: Side-effects of psychiatric medication impair quality of life and functioning. Furthermore, they contribute to morbidity, mortality, stigma, and poor treatment concordance resulting in relapse of psychiatric illness. Guidelines recommend discussing side-effects with patients when making treatment decisions, but a synthesis of antidepressant and antipsychotic side-effects to guide this process is missing, and considering all side-effects is a complex, multidimensional process. We aimed to create comprehensive databases of antipsychotic and antidepressant side-effects, and a digital tool to support database navigation.
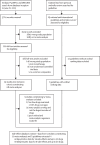
Methods: To create the databases, we did an umbrella review of Embase, PsycINFO, and MEDLINE from database inception to June 26, 2023. We included meta-analyses of randomised controlled trials examining antipsychotic monotherapy in the treatment of schizophrenia or antidepressant monotherapy in the treatment of major depressive disorder. We included meta-analyses in adults (aged ≥18 years) that assessed drugs with a common comparator. The search was complemented by a review of national and international guidelines and consensus statements for the treatment of major depressive disorder and schizophrenia in adults. Effect sizes for antipsychotic and antidepressant side-effects were extracted from meta-analyses examining the largest number of drugs. In cases of incomplete meta-analytic coverage, data were imputed on the basis of guideline-derived ordinal rankings or, if imputation was not possible, ordinal scores were extracted. Both meta-analytic and ordinal outcomes were normalised to provide values between 0 and 1. We then constructed a digital tool, the Psymatik Treatment Optimizer, to combine the side-effect databases with side-effect concerns of an individual user, to enable users to select side-effects of concern and the relative degree of concern for each side-effect. Concern weightings and the side-effect databases are synthesised via a multicriteria decision analysis method (technique for order of preference by similarity to ideal situation, or TOPSIS).
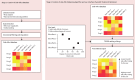
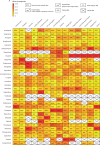
Findings: Of 3724 citations, 14 articles containing 68 meta-analyses of individual side-effects met inclusion criteria. After review of 19 guidelines, seven provided ordinal data. Antipsychotic data were extracted from five studies (11 meta-analyses, n=65 594 patients) and four guidelines, and antidepressant data were extracted from three guidelines. The resultant databases included data on 32 antipsychotics (14 side-effects) and 37 antidepressants (nine side-effects). The databases highlighted the clinical dilemma associated with balancing side-effects, with avoidance of one side-effect (eg, weight gain for antipsychotics) increasing the risk of others (eg, akathisia). To aid with this dilemma, the Psymatik Treatment Optimizer synthesises the side-effect databases with individual user-defined concern weights. After computing up to 5851 pairwise comparisons for antidepressants and 5142 pairwise comparisons for antipsychotics, Psymatik ranks treatments in order of preference for the individual user, with the output presented in a heatmap.
Interpretation: By facilitating collaborative, personalised, and evidence-based prescribing decisions, the side-effect databases and digital application supports care delivery that is consistent with international regulatory guidance for the treatment of schizophrenia and depression, and it therefore has promise for informing psychiatric practice and improving outcomes.
Funding: National Institute for Health and Care Research, Maudsley Charity, Wellcome Trust, Medical Research Council.
Copyright © 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests TP has participated in educational speaker meetings organised by Lundbeck, Otsuka, Sunovion, Janssen, Schwabe Pharma, ROVI Biotech, and Recordati. RAMcC has participated in advisory or speaker meetings organised by Otsuka, Karuna, Boehringer Ingelheim, and Janssen. TP and RAMcC are directors of Pharmatik that funds website hosting for Psymatik. Neither TP nor RAMcC have holdings or financial stakes in any pharmaceutical company. MH has received honoraria for advisory boards and lectures from Recordati. SJ has participated in educational speaker meetings organised by Lundbeck, Otsuka, Sunovion, Janssen, and Boehringer Ingelheim. FG has received honoraria from Lundbeck, Otsuka, Sunovion, and Boehringer Ingelheim. ODH has received investigator-initiated research funding from or participated in advisory or speaker meetings organised by Angellini, Autifony, Biogen, Boehringer Ingelheim, Eli Lilly, Heptares, Global Medical Education, Invicro, Janssen, Lundbeck, Neurocrine, Otsuka, Sunovion, Rand, Recordati, Roche, Viatris (formerly Mylan), and ROVI Biotech. AHY has delivered paid lectures and advisory boards for the following companies: AstraZeneca, Boehringer Ingelheim, Eli Lilly, LivaNova, Lundbeck, Sunovion, Servier, Livanova, Janssen, Allegan, Bionomics, Sumitomo Dainippon Pharma, COMPASS Pathways, Sage, Novartis, and Neurocentrx. CUC has been a consultant or advisor to or has received honoraria from AbbVie, Acadia, Alkermes, Allergan, Angelini, Aristo, Boehringer Ingelheim, Cardio Diagnostics, Cerevel, CNX Therapeutics, COMPASS Pathways, Darnitsa, Gedeon Richter, Hikma, Holmusk, IntraCellular Therapies, Janssen, Johnson & Johnson, Karuna, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Merck, Mindpax, Mitsubishi Tanabe Pharma, Mylan, Neurocrine, Newron, Noven, Novo Nordisk, Otsuka, Pharmabrain, PPD Biotech, Recordati, Relmada, Reviva, Rovi, Seqirus, SK Life Science, Sunovion, Sun Pharma, Supernus, Takeda, Teva, and Viatris; has provided expert testimony for Janssen and Otsuka; has served on a data safety monitoring board for COMPASS Pathways, Lundbeck, Relmada, Reviva, Rovi, Supernus, and Teva; has received grant support from Janssen and Takeda; has received royalties from UpToDate; and is a stock option holder of Cardio Diagnostics, MindPax, LB Pharma, and Quantic. All other authors declare no competing interests.
Figures







References
-
- Brody DJ, Gu Q. Antidepressant use among adults: United States, 2015–2018. National Center for Health Statistics data brief, no 377. 2020. https://www.cdc.gov/nchs/products/databriefs/db377.htm
-
- Public Health England Dependence and withdrawal associated with some prescribed medicines: an evidence review. 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploa...
-
- Lewer D, O'Reilly C, Mojtabai R, Evans-Lacko S. Antidepressant use in 27 European countries: associations with sociodemographic, cultural and economic factors. Br J Psychiatry. 2015;207:221–226. - PubMed
-
- Shoham N, Cooper C, Lewis G, Bebbington P, McManus S. Temporal trends in psychotic symptoms: repeated cross-sectional surveys of the population in England 2000–14. Schizophr Res. 2021;228:97–102. - PubMed
Uncited References
-
- Chung AK, Chua SE. Effects on prolongation of Bazett's corrected QT interval of seven second-generation antipsychotics in the treatment of schizophrenia: a meta-analysis. J Psychopharmacol. 2011;25:646–666. - PubMed
-
- Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951–962. - PubMed
-
- Bai Z, Wang G, Cai S, et al. Efficacy, acceptability and tolerability of 8 atypical antipsychotics in Chinese patients with acute schizophrenia: a network meta-analysis. Schizophr Res. 2017;185:73–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

