Brentuximab Vedotin-Driven Microtubule Disruption Results in Endoplasmic Reticulum Stress Leading to Immunogenic Cell Death and Antitumor Immunity
- PMID: 37775098
- PMCID: PMC10762337
- DOI: 10.1158/1535-7163.MCT-23-0118
Brentuximab Vedotin-Driven Microtubule Disruption Results in Endoplasmic Reticulum Stress Leading to Immunogenic Cell Death and Antitumor Immunity
Abstract
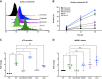
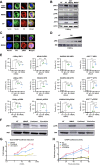
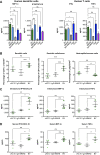
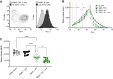
Brentuximab vedotin, a CD30-directed antibody-drug conjugate (ADC), is approved for clinical use in multiple CD30-expressing lymphomas. The cytotoxic payload component of brentuximab vedotin is monomethyl auristatin E (MMAE), a highly potent microtubule-disrupting agent. Preclinical results provided here demonstrate that treatment of cancer cells with brentuximab vedotin or free MMAE leads to a catastrophic disruption of the microtubule network eliciting a robust endoplasmic reticulum (ER) stress response that culminates in the induction of the classic hallmarks of immunogenic cell death (ICD). In accordance with the induction of ICD, brentuximab vedotin-killed lymphoma cells drove innate immune cell activation in vitro and in vivo. In the "gold-standard" test of ICD, vaccination of mice with brentuximab vedotin or free MMAE-killed tumor cells protected animals from tumor rechallenge; in addition, T cells transferred from previously vaccinated animals slowed tumor growth in immunodeficient mice. Immunity acquired from killed tumor cell vaccination was further amplified by the addition of PD-1 blockade. In a humanized model of CD30+ B-cell tumors, treatment with brentuximab vedotin drove the expansion and recruitment of autologous Epstein-Barr virus-reactive CD8+ T cells potentiating the activity of anti-PD-1 therapy. Together, these data support the ability of brentuximab vedotin and MMAE to drive ICD in tumor cells resulting in the activation of antigen-presenting cells and augmented T-cell immunity. These data provide a strong rationale for the clinical combination of brentuximab vedotin and other MMAE-based ADCs with checkpoint inhibitors.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures


References
-
- de Souza AP, Bonorino C. Tumor immunosuppressive environment: effects on tumor-specific and nontumor antigen immune responses. Expert Rev Anticancer Ther 2009;9:1317–32. - PubMed
-
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39:1–10. - PubMed
-
- Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat Immunol 2022;23:487–500. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

