Antitumor activity of the investigational B7-H3 antibody-drug conjugate, vobramitamab duocarmazine, in preclinical models of neuroblastoma
- PMID: 37775116
- PMCID: PMC10546160
- DOI: 10.1136/jitc-2023-007174
Antitumor activity of the investigational B7-H3 antibody-drug conjugate, vobramitamab duocarmazine, in preclinical models of neuroblastoma
Abstract
Introduction:
B7-H3 is a potential target for pediatric cancers, including neuroblastoma (NB). Vobramitamab duocarmazine (also referred to as MGC018 and herein referred to as vobra duo) is an investigational duocarmycin-based antibody-drug conjugate (ADC) directed against the B7-H3 antigen. It is composed of an anti-B7-H3 humanized IgG1/kappa monoclonal antibody chemically conjugated through a cleavable valine-citrulline linker to a
Methods: B7-H3 expression was evaluated by flow-cytometry in a panel of human NB cell lines. Cytotoxicity was evaluated in monolayer and in multicellular tumor spheroid (MCTS) models by the water-soluble tetrazolium salt,MTS, proliferation assay and Cell Titer Glo 3D cell viability assay, respectively. Apoptotic cell death was investigated by annexin V staining. Orthotopic, pseudometastatic, and resected mouse NB models were developed to mimic disease conditions related to primary tumor growth, metastases, and circulating tumor cells with minimal residual disease, respectively.
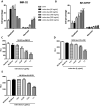
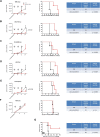
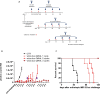
Results: All human NB cell lines expressed cell surface B7-H3 in a unimodal fashion. Vobra duo was cytotoxic in a dose-dependent and time-dependent manner against all cell lines (IC50 range 5.1-53.9 ng/mL) and NB MCTS (IC50 range 17.8-364 ng/mL). Vobra duo was inactive against a murine NB cell line (NX-S2) that did not express human B7-H3; however, NX-S2 cells were killed in the presence of vobra duo when co-cultured with human B7-H3-expressing cells, demonstrating bystander activity. In orthotopic and pseudometastatic mouse models, weekly intravenous treatments with 1 mg/kg vobra duo for 3 weeks delayed tumor growth compared with animals treated with an irrelevant (anti-CD20) duocarmycin-ADC. Vobra duo treatment for 4 weeks further increased survival in both orthotopic and resected NB models. Vobra duo compared favorably to TOpotecan-TEMozolomide (TOTEM), the standard-of-care therapy for NB relapsed disease, with tumor relapse delayed or arrested by two or three repeated 4-week vobra duo treatments, respectively. Further increased survival was observed in mice treated with vobra duo in combination with TOTEM. Vobra duo treatment was not associated with body weight loss, hematological toxicity, or clinical chemistry abnormalities.
Conclusion: Vobra duo exerts relevant antitumor activity in preclinical B7-H3-expressing NB models and represents a potential candidate for clinical translation.
Keywords: drug evaluation, preclinical; immunotherapy; neuroblastoma.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: EB and DL are employees of MacroGenics and receive salary and stocks as part of their compensations. The other authors do not have competing interests to declare.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
