Internet-delivered cognitive behavioural therapy programme to reduce depressive symptoms in patients with multiple sclerosis: a multicentre, randomised, controlled, phase 3 trial
- PMID: 37775187
- PMCID: PMC10921847
- DOI: 10.1016/S2589-7500(23)00109-7
Internet-delivered cognitive behavioural therapy programme to reduce depressive symptoms in patients with multiple sclerosis: a multicentre, randomised, controlled, phase 3 trial
Abstract
Background: Depression is three to four times more prevalent in patients with neurological and inflammatory disorders than in the general population. For example, in patients with multiple sclerosis, the 12-month prevalence of major depressive disorder is around 25% and it is associated with a lower quality of life, faster disease progression, and higher morbidity and mortality. Despite its clinical relevance, there are few treatment options for depression associated with multiple sclerosis and confirmatory trials are scarce. We aimed to evaluate the safety and efficacy of a multiple sclerosis-specific, internet-based cognitive behavioural therapy (iCBT) programme for the treatment of depressive symptoms associated with the disease.
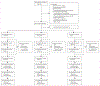
Methods: This parallel-group, randomised, controlled, phase 3 trial of an iCBT programme to reduce depressive symptoms in patients with multiple sclerosis was carried out at five academic centres with large outpatient care units in Germany and the USA. Patients with a neurologist-confirmed diagnosis of multiple sclerosis and depressive symptoms were randomly assigned (1:1:1; automated assignment, concealed allocation, no stratification, no blocking) to receive treatment as usual plus one of two versions of the iCBT programme Amiria (stand-alone or therapist-guided) or to a control condition, in which participants received treatment as usual and were offered access to the iCBT programme after 6 months. Masking of participants to group assignment between active treatment and control was not possible, although raters were masked to group assignment. The predefined primary endpoint, which was analysed in the intention-to-treat population, was severity of depressive symptoms as measured by the Beck Depression Inventory-II (BDI-II) at week 12 after randomisation. This trial is registered at ClinicalTrials.gov, NCT02740361, and is complete.
Findings: Between May 3, 2017, and Nov 4, 2020, we screened 485 patients for eligibility. 279 participants were enrolled, of whom 101 were allocated to receive stand-alone iCBT, 85 to receive guided iCBT, and 93 to the control condition. The dropout rate at week 12 was 18% (50 participants). Both versions of the iCBT programme significantly reduced depressive symptoms compared with the control group (BDI-II between-group mean differences: control vs stand-alone iCBT 6·32 points [95% CI 3·37-9·27], p<0·0001, effect size d=0·97 [95% CI 0·64-1·30]; control vs guided iCBT 5·80 points [2·71-8·88], p<0·0001, effect size d=0·96 [0·62-1·30]). Clinically relevant worsening of depressive symptoms was observed in three participants in the control group, one in the stand-alone iCBT group, and none in the guided iCBT group. No occurrences of suicidality were observed during the trial and there were no deaths.
Interpretation: This trial provides evidence for the safety and efficacy of a multiple sclerosis-specific iCBT tool to reduce depressive symptoms in patients with the disease. This remote-access, scalable intervention increases the therapeutic options in this patient group and could help to overcome treatment barriers.
Funding: National Multiple Sclerosis Society (USA).
Copyright © 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests SMG reports honoraria from Hexal and research grants from Biogen, the German Ministry for Education and Research, the German Ministry of Health, Deutsche Forschungsgemeinschaft, and the National Multiple Sclerosis Society (NMSS). TF reports personnel fees for consultancies (including data monitoring committees and advisory boards) from Bayer, Bristol Myers Squibb, CSL Behring, Enanta Pharmaceuticals, Fresenius Kabi, Galapagos, Johnson & Johnson, Janssen Pharmaceuticals, LivaNova, Minoryx Therapeutics, Novartis, Roche, Vifor Pharma, Immunic Therapeutics, and Kyowa Kirin. BM is an employee of the GAIA Group, the owner and distributor of commercial digital health interventions including Deprexis and the multiple sclerosis-specific internet-delivered cognitive behavioural therapy Amiria. RM-M reports personal fees from training in cognitive behavioural therapy for irritable bowel syndrome for Central and North West London NHS Foundation Trust and University of Southampton, outside the submitted work. She receives payment for consultancy to Mahana Therapeutics, has share options in Mahana Therapeutics, and is a beneficiary of a licence agreement between Mahana Therapeutics and King's College London. SGL has participated in numerous clinical trials, receiving research support from Roche, Biogen, TG Therapeutics, Novartis, Bristol-Myers Squibb, Atara Biotherapeutics, Anokion, Immunic Therapeutics, Sanofi, and the Patient-Centered Outcomes Research Institute; has received support from NMSS-Investigator initiated studies on multiple sclerosis wellness and rehabilitation; and has unpaid board positions for the Friends of the Multiple Sclerosis Achievement Center and the Mid America Chapter of the NMSS. CR reports a stipend from Förderfond der Medizinischen Fakultät, Universitätsklinikum Hamburg Eppendorf. I-KP declares grants from Deutsche Multiple Sklerose Gesellschaft, Novartis, Teva Pharmaceuticals, and Roche; payment or honoraria from Almirall, Bayer Pharma, Biogen, BMS, Celgene, Genzyme, Janssen Pharmaceuticals, Merck, Novartis, Roche, and Teva Pharmaceuticals; and participation on data safety monitoring boards or advisory boards for Biogen, BMS, Janssen Pharmaceuticals, Merck, Novartis, and Roche. FP reports grants from the German Ministry for Education and Research, Deutsche Forschungsgemeinschaft, the Einstein Foundation, the Guthy Jackson Charitable Foundation, the EU FP7 Framework Program, Biogen, Genzyme, Merck Serono, Novartis, Bayer, Roche, Parexel, and Almirall; payment or honoraria from the Guthy Jackson Foundation, Bayer, Biogen, Merck Serono, Sanofi Genzyme, Novartis, Viela Bio, Roche, Union Chimique Belge (UCB), Mitsubishi Tanabe, and Celgene; support for attending meetings or travel from Guthy Jackson Foundation, Bayer, Biogen, Merck Serono, Sanofi Genzyme, Novartis, Alexion, Viela Bio, Roche, UCB, Mitsubishi Tanabe, and Celgene; participation on data safety monitoring boards or advisory boards for Celgene, Roche, UCB, and Merck; and unpaid editor positions for PLoS One and Neurology Neuroimmunology & Neuroinflammation. NLS receives research grants from the National Institutes of Health National Institute of Neurological Disorders and Stroke and a leadership role as Chair of the National Medical Advisory Committee of the NMSS. JMB has served on the Novartis unbranded speakers’ bureau and has received grant support from Genzyme. PAA has served on the EMD Serono speakers’ bureau and served as a consultant for Biogen and Roche Pharmaceuticals. CH reports payment or honoraria for presentations from Roche. All other authors declare no competing interests.
Figures

Comment in
-
Early intervention for depressive symptoms in multiple sclerosis.Lancet Digit Health. 2023 Oct;5(10):e637-e638. doi: 10.1016/S2589-7500(23)00162-0. Lancet Digit Health. 2023. PMID: 37775184 No abstract available.
References
-
- Gold SM, Köhler-Forsberg O, Moss-Morris R, et al. Comorbid depression in medical diseases. Nat Rev Dis Primers 2020; 6: 69. - PubMed
-
- Marrie RA. Comorbidity in multiple sclerosis: implications for patient care. Nat Rev Neurol 2017; 13: 375–82. - PubMed
-
- Boeschoten RE, Braamse AMJ, Beekman ATF, et al. Prevalence of depression and anxiety in multiple sclerosis: a systematic review and meta-analysis. J Neurol Sci 2017; 372: 331–41. - PubMed

