Pattern of Brain Parenchymal Damage Related to Cerebral Small Vessel Disease in Carriers of Rare NOTCH3 Variants
- PMID: 37775315
- PMCID: PMC10662991
- DOI: 10.1212/WNL.0000000000207882
Pattern of Brain Parenchymal Damage Related to Cerebral Small Vessel Disease in Carriers of Rare NOTCH3 Variants
Abstract
Background and objectives: Previous studies reported that carriers of rare NOTCH3 variants comprised more than 10% of the general population and are susceptible to a heavy overall burden of cerebral small vessel disease while the injury patterns remain uncovered. This study aimed to investigate the imaging features in relation to rare NOTCH3 variants and the interaction between cortical atrophy and white matter lesions from a longitudinal view, with respect to spatial and dynamic patterns.
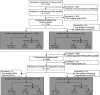
Methods: As part of a community-based cohort, we included participants with complete whole-exome sequencing and brain MRI in the baseline analysis. All participants were invited for a 5-year follow-up MRI, and those who did not complete the follow-up were excluded from the longitudinal analysis. NOTCH3 variants with minor allele frequency <1% in all 4 public population databases were defined as rare variants. We used general linear models to compare the volume of white matter hyperintensity (WMH) volume and brain parenchymal fraction between rare NOTCH3 variant carriers and noncarriers. In addition, we compared the WMH probability map and vertex-wise cortex maps at a voxel/vertex-wise level.
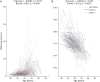
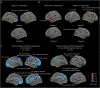
Results: A total of 1,054 participants were included in baseline analysis (13.56% carried rare NOTCH3 variants), among whom 661 had a follow-up brain MRI (13.76% carried rare NOTCH3 variants). Rare NOTCH3 variant carriers had a heavier white matter hyperintensity burden (1.65 vs 0.85 mL, p = 0.025) and had more extensive WMH distributed in the periventricular areas. We also found that rare NOTCH3 variant carriers were susceptible to worse cortical atrophy (β = -0.004, SE = 0.002, p = 0.057, adjusted for age and sex). Cortical atrophy of multiple regions in the frontal and parietal lobes was related to white matter hyperintensity progression.
Discussion: Individuals with rare NOTCH3 variants have a distinct pattern of brain parenchymal damage related to CSVD. Our findings uncover the important genetic predisposition in age-related cerebral small vessel disease in the general population.
© 2023 American Academy of Neurology.
Conflict of interest statement
The authors report no relevant disclosures. Go to
Figures

Similar articles
-
Rare NOTCH3 Variants in a Chinese Population-Based Cohort and Its Relationship With Cerebral Small Vessel Disease.Stroke. 2021 Dec;52(12):3918-3925. doi: 10.1161/STROKEAHA.120.032265. Epub 2021 Aug 18. Stroke. 2021. PMID: 34404235
-
Association of variants in HTRA1 and NOTCH3 with MRI-defined extremes of cerebral small vessel disease in older subjects.Brain. 2019 Apr 1;142(4):1009-1023. doi: 10.1093/brain/awz024. Brain. 2019. PMID: 30859180 Free PMC article.
-
Genetic variants of the NOTCH3 gene in the elderly and magnetic resonance imaging correlates of age-related cerebral small vessel disease.Brain. 2011 Nov;134(Pt 11):3384-97. doi: 10.1093/brain/awr252. Epub 2011 Oct 17. Brain. 2011. PMID: 22006983 Free PMC article.
-
Association of NOTCH3 Variant Risk Category With 2-Year Clinical and Radiologic Small Vessel Disease Progression in Patients With CADASIL.Neurology. 2024 May 28;102(10):e209310. doi: 10.1212/WNL.0000000000209310. Epub 2024 May 7. Neurology. 2024. PMID: 38713890 Free PMC article.
-
Pathophysiology of cerebral small vessel disease: a journey through recent discoveries.J Clin Invest. 2024 May 15;134(10):e172841. doi: 10.1172/JCI172841. J Clin Invest. 2024. PMID: 38747292 Free PMC article. Review.
Cited by
-
The Kashima Scan Study 2: a protocol for a prospective observational cohort study of cerebral small vessel disease in neurologically healthy adults.Environ Health Prev Med. 2025;30:52. doi: 10.1265/ehpm.25-00135. Environ Health Prev Med. 2025. PMID: 40603063 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
