New drug discovery of cardiac anti-arrhythmic drugs: insights in animal models
- PMID: 37775650
- PMCID: PMC10541452
- DOI: 10.1038/s41598-023-41942-4
New drug discovery of cardiac anti-arrhythmic drugs: insights in animal models
Abstract
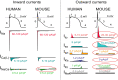
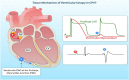
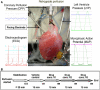
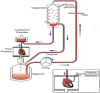
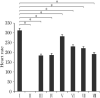
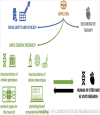
Cardiac rhythm regulated by micro-macroscopic structures of heart. Pacemaker abnormalities or disruptions in electrical conduction, lead to arrhythmic disorders may be benign, typical, threatening, ultimately fatal, occurs in clinical practice, patients on digitalis, anaesthesia or acute myocardial infarction. Both traditional and genetic animal models are: In-vitro: Isolated ventricular Myocytes, Guinea pig papillary muscles, Patch-Clamp Experiments, Porcine Atrial Myocytes, Guinea pig ventricular myocytes, Guinea pig papillary muscle: action potential and refractory period, Langendorff technique, Arrhythmia by acetylcholine or potassium. Acquired arrhythmia disorders: Transverse Aortic Constriction, Myocardial Ischemia, Complete Heart Block and AV Node Ablation, Chronic Tachypacing, Inflammation, Metabolic and Drug-Induced Arrhythmia. In-Vivo: Chemically induced arrhythmia: Aconitine antagonism, Digoxin-induced arrhythmia, Strophanthin/ouabain-induced arrhythmia, Adrenaline-induced arrhythmia, and Calcium-induced arrhythmia. Electrically induced arrhythmia: Ventricular fibrillation electrical threshold, Arrhythmia through programmed electrical stimulation, sudden coronary death in dogs, Exercise ventricular fibrillation. Genetic Arrhythmia: Channelopathies, Calcium Release Deficiency Syndrome, Long QT Syndrome, Short QT Syndrome, Brugada Syndrome. Genetic with Structural Heart Disease: Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia, Dilated Cardiomyopathy, Hypertrophic Cardiomyopathy, Atrial Fibrillation, Sick Sinus Syndrome, Atrioventricular Block, Preexcitation Syndrome. Arrhythmia in Pluripotent Stem Cell Cardiomyocytes. Conclusion: Both traditional and genetic, experimental models of cardiac arrhythmias' characteristics and significance help in development of new antiarrhythmic drugs.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Roden DM. Antiarrhythmie Drugs. In: Laurence LB, Lazo JS, Parker KL, editors. Goodman & Gilman's The Pharmcological Basis of Thaerapeutics. 11. McGraw Hill; 2006. pp. 899–932.
-
- Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The cardiac arrhythmia suppression trial. N. Engl. J. Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

