Second breast cancer: recurrence score results, clinicopathologic characteristics, adjuvant treatments, and outcomes-exploratory analysis of the Clalit registry
- PMID: 37775723
- PMCID: PMC10541873
- DOI: 10.1038/s41523-023-00586-3
Second breast cancer: recurrence score results, clinicopathologic characteristics, adjuvant treatments, and outcomes-exploratory analysis of the Clalit registry
Abstract
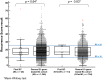
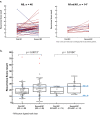
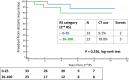
Data on using the 21-gene Recurrence Score (RS) testing on second breast cancer (BC; second primary or local recurrence) are lacking. This cohort study examined patients with first and second BC, who underwent 21-gene testing both times. It included a 'study-cohort' (60 N0/N1mi/N1 ER + HER2‒ BC patients with ≥2 RS results >1 year apart) and a 'general 21-gene-tested BC-cohort' (2044 previously described N0/N1mi/N1 patients). The median time between the first and second BC was 5.2 (IQR, 3.1-7.1) years; the second BC was ipsilateral in 68%. Patient/tumor characteristics of the first- and second-BC in the 'study-cohort' were similar, except for the RS which was higher in the second BC (median [IQR]: 23 [17-30] vs 17 [14-22], p < 0.001). Overall, 56 patients had follow-up data, of whom 5 experienced distant recurrence (2 RS 11-25 patients and 3 RS 26-100 patients). Studies exploring the prognostic utility of the RS in this setting are warranted.
© 2023. The Author(s).
Conflict of interest statement
The authors declared the following competing financial interests and no nonfinancial interests. L.S-G. is employed by and owns stock at Oncotest-Rhenium. A.B-S. is a consultant for Oncotest-Rhenium, S.P-S. reports receiving honoraria from Exact Sciences. All other authors declare no competing financial or non-financial interests.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

