Rate of response to initial antiretroviral therapy according to level of pre-existing HIV-1 drug resistance detected by next-generation sequencing in the strategic timing of antiretroviral treatment (START) study
- PMID: 37775947
- PMCID: PMC10872720
- DOI: 10.1111/hiv.13556
Rate of response to initial antiretroviral therapy according to level of pre-existing HIV-1 drug resistance detected by next-generation sequencing in the strategic timing of antiretroviral treatment (START) study
Abstract
Objectives: The main objective of this analysis was to evaluate the impact of pre-existing drug resistance by next-generation sequencing (NGS) on the risk of treatment failure (TF) of first-line regimens in participants enrolled in the START study.
Methods: Stored plasma from participants with entry HIV RNA >1000 copies/mL were analysed using NGS (llumina MiSeq). Pre-existing drug resistance was defined using the mutations considered by the Stanford HIV Drug Resistance Database (HIVDB v8.6) to calculate the genotypic susceptibility score (GSS, estimating the number of active drugs) for the first-line regimen at the detection threshold windows of >20%, >5%, and >2% of the viral population. Survival analysis was conducted to evaluate the association between the GSS and risk of TF (viral load >200 copies/mL plus treatment change).
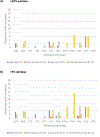
Results: Baseline NGS data were available for 1380 antiretroviral therapy (ART)-naïve participants enrolled over 2009-2013. First-line ART included a non-nucleoside reverse transcriptase inhibitor (NNRTI) in 976 (71%), a boosted protease inhibitor in 297 (22%), or an integrase strand transfer inhibitor in 107 (8%). The proportions of participants with GSS <3 were 7% for >20%, 10% for >5%, and 17% for the >2% thresholds, respectively. The adjusted hazard ratio of TF associated with a GSS of 0-2.75 versus 3 in the subset of participants with mutations detected at the >2% threshold was 1.66 (95% confidence interval 1.01-2.74; p = 0.05) and 2.32 (95% confidence interval 1.32-4.09; p = 0.003) after restricting the analysis to participants who started an NNRTI-based regimen.
Conclusions: Up to 17% of participants initiated ART with a GSS <3 on the basis of NGS data. Minority variants were predictive of TF, especially for participants starting NNRTI-based regimens.
Keywords: HIV drug resistance; antiretroviral therapy; human immunodeficiency virus (HIV); next generation sequencing.
© 2023 The Authors. HIV Medicine published by John Wiley & Sons Ltd on behalf of British HIV Association.
Conflict of interest statement
ACL declares no conflicts of interest.
Figures




References
-
- Johnson JA, Li JF, Wei X, Lipscomb J, Irlbeck D, Craig C, Smith A, Bennett DE, Monsour M, Sandstrom P, Lanier ER, Heneine W. Minority HIV-1 drug resistance mutations are present in antiretroviral treatment-naïve populations and associate with reduced treatment efficacy. PLoS Med. 2008. Jul 29;5(7):e158. doi: 10.1371/journal.pmed.0050158. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

