Call to action: a five nations consensus on the use of intravenous zoledronate after hip fracture
- PMID: 37776543
- PMCID: PMC10542103
- DOI: 10.1093/ageing/afad172
Call to action: a five nations consensus on the use of intravenous zoledronate after hip fracture
Erratum in
-
Correction to: Call to action: a five nations consensus on the use of intravenous zoledronate after hip fracture.Age Ageing. 2024 Jan 2;53(1):afae014. doi: 10.1093/ageing/afae014. Age Ageing. 2024. PMID: 38261453 Free PMC article. No abstract available.
Abstract
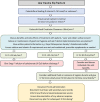
Currently in the UK and Ireland, after a hip fracture most patients do not receive bone protection medication to reduce the risk of refracture. Yet randomised controlled trial data specifically examining patients with hip fracture have shown that intravenous zoledronate reduces refracture risk by a third. Despite this evidence, use of intravenous zoledronate is highly variable following a hip fracture; many hospitals are providing this treatment, whilst most are currently not. A range of clinical uncertainties, doubts over the evidence base and practical concerns are cited as reasons. This paper discusses these concerns and provides guidance from expert consensus, aiming to assist orthogeriatricians, pharmacists and health services managers establish local protocols to deliver this highly clinically and cost-effective treatment to patients before they leave hospital, in order to reduce costly re-fractures in this frail population.
Keywords: hip fracture; older people; osteoporosis; secondary prevention; zoledronate.
© The Author(s) 2023. Published by Oxford University Press on behalf of the British Geriatrics Society. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
All eight authors have completed and submitted the declaration of interest forms used by NICE for its advisory committees. These identify that M.K.J. is an advisory group member, that O.S. and M.K.J. have received speaker fees, that M.K.J. has received institutional grant funding, and consultancy fees and that A.J.B. and M.K.J. have received support for conference registration, accommodation and travel from various drug companies. However, none of these companies are the manufacturers of Aclasta or of generic forms of zoledronate. The authors’ other declarations, of non-financial, professional or personal interest reflect their voluntary roles in the national and international organisation that are described in the Methodology section; including A.J., A.M. and E.A.’s roles as leads of the hip fracture audits for the five nations of the British Isles.
Figures
Comment in
-
Zoledronate for hip fractures: the road towards achieving consensus.Age Ageing. 2024 Apr 1;53(4):afae062. doi: 10.1093/ageing/afae062. Age Ageing. 2024. PMID: 38557668 No abstract available.
References
-
- Royal College of Physicians . 15 years of quality improvement: the 2023 National Hip Fracture Database report on 2022. London: RCP, 2023.
-
- The National Osteoporosis Guideline Group. NOGG . Clinical guideline for the prevention and treatment of osteoporosis. 2021; 24–8. www.nogg.org.uk.
-
- Scottish Standards of Care for Hip Fracture , 2023. www.shfa.scot.nhs.uk (March 2023, date last accessed).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

