Small molecule screen identifies pyrimethamine as an inhibitor of NRF2-driven esophageal hyperplasia
- PMID: 37776708
- PMCID: PMC10558795
- DOI: 10.1016/j.redox.2023.102901
Small molecule screen identifies pyrimethamine as an inhibitor of NRF2-driven esophageal hyperplasia
Abstract
Objective: NRF2 is a master transcription factor that regulates the stress response. NRF2 is frequently mutated and activated in human esophageal squamous cell carcinoma (ESCC), which drives resistance to chemotherapy and radiation therapy. Therefore, a great need exists for NRF2 inhibitors for targeted therapy of NRF2high ESCC.
Design: We performed high-throughput screening of two compound libraries from which hit compounds were further validated in human ESCC cells and a genetically modified mouse model. The mechanism of action of one compound was explored by biochemical assays.
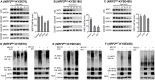
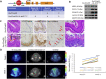
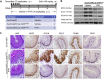
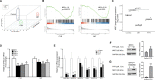
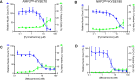
Results: Using high-throughput screening of two small molecule compound libraries, we identified 11 hit compounds as potential NRF2 inhibitors with minimal cytotoxicity at specified concentrations. We then validated two of these compounds, pyrimethamine and mitoxantrone, by demonstrating their dose- and time-dependent inhibitory effects on the expression of NRF2 and its target genes in two NRF2Mut human ESCC cells (KYSE70 and KYSE180). RNAseq and qPCR confirmed the suppression of global NRF2 signaling by these two compounds. Mechanistically, pyrimethamine reduced NRF2 half-life by promoting NRF2 ubiquitination and degradation in KYSE70 and KYSE180 cells. Expression of an Nrf2E79Q allele in mouse esophageal epithelium (Sox2CreER;LSL-Nrf2E79Q/+) resulted in an NRF2high phenotype, which included squamous hyperplasia, hyperkeratinization, and hyperactive glycolysis. Treatment with pyrimethamine (30 mg/kg/day, p.o.) suppressed the NRF2high esophageal phenotype with no observed toxicity.
Conclusion: We have identified and validated pyrimethamine as an NRF2 inhibitor that may be rapidly tested in the clinic for NRF2high ESCC.
Keywords: Esophageal squamous cell carcinoma; KEAP1; Mitoxantrone; NRF2; Pyrimethamine.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no potential conflicts of interest.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

