Oral fungal profiling and risk of nasopharyngeal carcinoma: a population-based case-control study
- PMID: 37776725
- PMCID: PMC10550808
- DOI: 10.1016/j.ebiom.2023.104813
Oral fungal profiling and risk of nasopharyngeal carcinoma: a population-based case-control study
Abstract
Background: Dysbiosis of the oral mycobiome has been linked to some diseases, including cancers. However, the role of oral fungal communities in nasopharyngeal carcinoma (NPC) carcinogenesis has not previously been investigated.
Methods: We characterized the oral salivary fungal mycobiome in 476 untreated incident NPC patients and 537 population-based controls using fungal internal transcribed spacer (ITS)-2 sequencing. The relationship between oral fungal mycobiome and the risk of NPC was assessed through bioinformatic and biostatistical analyses.
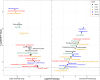
Findings: We found that lower fungal alpha diversity was associated with an increased odds of NPC [lower vs. higher: observed features (adjusted odds ratio [OR] = 5.81, 95% confidence interval [CI] = 3.60-9.38); Simpson diversity (1.53, 1.03-2.29); Shannon diversity (2.03, 1.35-3.04)]. We also observed a significant difference in global fungal community patterns between cases and controls based on Bray-Curtis dissimilarity (P < 0.001). Carriage of oral fungal species, specifically, Saccharomyces cerevisiae, Candida tropicalis, Lodderomyces elongisporus, Candida albicans, and Fusarium poae, was associated with significantly higher odds of NPC, with ORs ranging from 1.56 to 4.66. Individuals with both low fungal and low bacterial alpha diversity had a profoundly elevated risk of NPC.
Interpretation: Our results suggest that dysbiosis in the oral mycobiome, characterized by a loss of fungal community diversity and overgrowth of several fungal organisms, is associated with a substantially increased risk of NPC.
Funding: This work was funded by the US National Institutes of Health, the Swedish Research Council, the High-level Talents Research Start-up Project of Fujian Medical University, and the China Scholarship Council.
Keywords: Case–control study; Fungi; ITS sequencing; Microbiome; Nasopharyngeal carcinoma; Oral mycobiome.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests E.C. is an employee of GRAIL, LLC, which played no role in the development of this manuscript. The other authors declare no competing interests.
Figures


References
-
- Nilsson R.H., Anslan S., Bahram M., Wurzbacher C., Baldrian P., Tedersoo L. Mycobiome diversity: high-throughput sequencing and identification of fungi. Nat Rev Microbiol. 2018;17:95–109. - PubMed
-
- Bandara H.M.H.N., Panduwawala C.P., Samaranayake L.P. Biodiversity of the human oral mycobiome in health and disease. Oral Dis. 2019;25:363–371. - PubMed

