Iron acquisition by a commensal bacterium modifies host nutritional immunity during Salmonella infection
- PMID: 37776864
- PMCID: PMC10599249
- DOI: 10.1016/j.chom.2023.08.018
Iron acquisition by a commensal bacterium modifies host nutritional immunity during Salmonella infection
Abstract
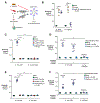
During intestinal inflammation, host nutritional immunity starves microbes of essential micronutrients, such as iron. Pathogens scavenge iron using siderophores, including enterobactin; however, this strategy is counteracted by host protein lipocalin-2, which sequesters iron-laden enterobactin. Although this iron competition occurs in the presence of gut bacteria, the roles of commensals in nutritional immunity involving iron remain unexplored. Here, we report that the gut commensal Bacteroides thetaiotaomicron acquires iron and sustains its resilience in the inflamed gut by utilizing siderophores produced by other bacteria, including Salmonella, via a secreted siderophore-binding lipoprotein XusB. Notably, XusB-bound enterobactin is less accessible to host sequestration by lipocalin-2 but can be "re-acquired" by Salmonella, allowing the pathogen to evade nutritional immunity. Because the host and pathogen have been the focus of studies of nutritional immunity, this work adds commensal iron metabolism as a previously unrecognized mechanism modulating the host-pathogen interactions and nutritional immunity.
Keywords: Salmonella; commensal iron metabolism; enteric pathogen; gut microbiota resilience; intestinal inflammation; nutritional immunity; siderophore.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




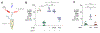

Update of
-
Iron acquisition by a commensal bacterium modifies host nutritional immunity during Salmonella infection.bioRxiv [Preprint]. 2023 Jun 26:2023.06.25.546471. doi: 10.1101/2023.06.25.546471. bioRxiv. 2023. Update in: Cell Host Microbe. 2023 Oct 11;31(10):1639-1654.e10. doi: 10.1016/j.chom.2023.08.018. PMID: 37425782 Free PMC article. Updated. Preprint.
Comment in
-
Robbing the thief.Cell Host Microbe. 2023 Oct 11;31(10):1597-1599. doi: 10.1016/j.chom.2023.09.009. Cell Host Microbe. 2023. PMID: 37827119
References
Key Resource Table Reference:
-
- Hoiseth SK and Stocker BA, Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature, 1981. 291(5812): p. 238–9. - PubMed
MeSH terms
Substances
Grants and funding
- S10 RR023748/RR/NCRR NIH HHS/United States
- R01 DK131104/DK/NIDDK NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- F31 AI172352/AI/NIAID NIH HHS/United States
- R01 AI164587/AI/NIAID NIH HHS/United States
- S10 RR026992/RR/NCRR NIH HHS/United States
- R01 AI168302/AI/NIAID NIH HHS/United States
- F31 AI178950/AI/NIAID NIH HHS/United States
- T32 AI112541/AI/NIAID NIH HHS/United States
- T32 ES007028/ES/NIEHS NIH HHS/United States
- R01 AI101171/AI/NIAID NIH HHS/United States
- R35 GM147470/GM/NIGMS NIH HHS/United States
- R01 DK134692/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

