LDL binding to cell receptors and extracellular matrix is proatherogenic in obesity but improves after bariatric surgery
- PMID: 37777014
- PMCID: PMC10665669
- DOI: 10.1016/j.jlr.2023.100451
LDL binding to cell receptors and extracellular matrix is proatherogenic in obesity but improves after bariatric surgery
Abstract
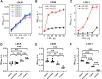
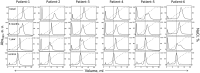
Obesity is a major global public health issue involving dyslipidemia, oxidative stress, inflammation, and increased risk of CVD. Weight loss reduces this risk, but the biochemical underpinnings are unclear. We explored how obesity and weight loss after bariatric surgery influence LDL interactions that trigger proatherogenic versus antiatherogenic processes. LDL was isolated from plasma of six patients with severe obesity before (basal) and 6-12 months after bariatric surgery (basal BMI = 42.7 kg/m2; 6-months and 12-months postoperative BMI = 34.1 and 30 kg/m2). Control LDL were from six healthy subjects (BMI = 22.6 kg/m2). LDL binding was quantified by ELISA; LDL size and charge were assessed by chromatography; LDL biochemical composition was determined. Compared to controls, basal LDL showed decreased nonatherogenic binding to LDL receptor, which improved postoperatively. Conversely, basal LDL showed increased binding to scavenger receptors LOX1 and CD36 and to glycosaminoglycans, fibronectin and collagen, which is proatherogenic. One year postoperatively, this binding decreased but remained elevated, consistent with elevated lipid peroxidation. Serum amyloid A and nonesterified fatty acids were elevated in basal and postoperative LDL, indicating obesity-associated inflammation. Aggregated and electronegative LDL remained elevated, suggesting proatherogenic processes. These results suggest that obesity-induced inflammation contributes to harmful LDL alterations that probably increase the risk of CVD. We conclude that in obesity, LDL interactions with cell receptors and extracellular matrix shift in a proatherogenic manner but are partially reversed upon postoperative weight loss. These results help explain why the risk of CVD increases in obesity but decreases upon weight loss.
Keywords: CD36 and LOX-1; Inflammation in obesity; LDL binding to LDL receptor; LDL retention by matrix proteins and glycosaminoglycans; Lipoprotein lipolysis; oxidation and aggregation.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures




References
-
- Chooi Y.C., Ding C., Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. - PubMed
-
- Grundy S.M. Obesity, metabolic syndrome, and cardiovascular disease. J. Clin. Endocrinol. Metab. 2004;89:2595–2600. - PubMed
-
- Poirier P., Giles T.D., Bray G.A., Hong Y., Stern J.S., Pi-Sunyer F.X., et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific statement on obesity and heart disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. - PubMed
-
- Bratti L.D.O.S., Do Carmo Í.A.R., Vilela T.F., Souza L.C., Moraes A.C.R.D., Filippin-Monteiro F.B. Bariatric surgery improves clinical outcomes and adiposity biomarkers but not inflammatory cytokines SAA and MCP-1 after a six-month follow-up. Scand. J. Clin. Lab. Invest. 2021;81:230–236. - PubMed
-
- Franssen R., Monajemi H., Stroes E.S.G., Kastelein J.J.P. Obesity and dyslipidemia. Med. Clin. North Am. 2011;95:893–902. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

