Metaorganismal choline metabolism shapes olfactory perception
- PMID: 37777156
- PMCID: PMC10630631
- DOI: 10.1016/j.jbc.2023.105299
Metaorganismal choline metabolism shapes olfactory perception
Abstract
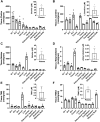
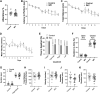
Microbes living in the intestine can regulate key signaling processes in the central nervous system that directly impact brain health. This gut-brain signaling axis is partially mediated by microbe-host-dependent immune regulation, gut-innervating neuronal communication, and endocrine-like small molecule metabolites that originate from bacteria to ultimately cross the blood-brain barrier. Given the mounting evidence of gut-brain crosstalk, a new therapeutic approach of "psychobiotics" has emerged, whereby strategies designed to primarily modify the gut microbiome have been shown to improve mental health or slow neurodegenerative diseases. Diet is one of the most powerful determinants of gut microbiome community structure, and dietary habits are associated with brain health and disease. Recently, the metaorganismal (i.e., diet-microbe-host) trimethylamine N-oxide (TMAO) pathway has been linked to the development of several brain diseases including Alzheimer's, Parkinson's, and ischemic stroke. However, it is poorly understood how metaorganismal TMAO production influences brain function under normal physiological conditions. To address this, here we have reduced TMAO levels by inhibiting gut microbe-driven choline conversion to trimethylamine (TMA), and then performed comprehensive behavioral phenotyping in mice. Unexpectedly, we find that TMAO is particularly enriched in the murine olfactory bulb, and when TMAO production is blunted at the level of bacterial choline TMA lyase (CutC/D), olfactory perception is altered. Taken together, our studies demonstrate a previously underappreciated role for the TMAO pathway in olfactory-related behaviors.
Keywords: Nutrition; gut microbiome; metabolism; olfaction.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest W. J. M., K. E. K., T. C. J., A. H., S. C., L. J. O., R. B., D. J. S., A. B., A. L. B., O. R., J. D. L., and J. M. B. all declare no competing financial interests. Z. W. and S. L. H. report being named as co-inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. S. L. H. also reports being a paid consultant for Zehna Therapeutics. S. L. H reports having received research funds from Procter & Gamble, Zehna Therapeutics and Roche Diagnostics. Z. W. and S. L. H. report being eligible to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland Heart Lab, and Procter & Gamble, and S. L. H. from Zehna Therapeutics.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

