Crotonylated BEX2 interacts with NDP52 and enhances mitophagy to modulate chemotherapeutic agent-induced apoptosis in non-small-cell lung cancer cells
- PMID: 37777549
- PMCID: PMC10542755
- DOI: 10.1038/s41419-023-06164-6
Crotonylated BEX2 interacts with NDP52 and enhances mitophagy to modulate chemotherapeutic agent-induced apoptosis in non-small-cell lung cancer cells
Abstract
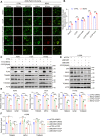
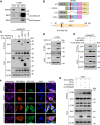
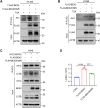
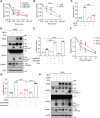
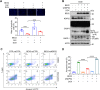
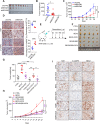
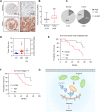
Brain expressed X-linked gene 2 (BEX2) encoded protein was originally identified to promote transcription by interacting with several transcription factors in the DNA-binding complexes. Recently, BEX2 was found to be localized in cytosol and/or mitochondria and regulate apoptosis in cancer cells and tumor growth. However, the molecular mechanism underlying its roles in cancer cells remains unclear. Here, we report that crotonylated BEX2 plays an important role in inhibiting chemotherapeutic agent-induced apoptosis via enhancing mitophagy in human lung cancer cells. BEX2 promotes mitophagy by facilitating interaction between NDP52 and LC3B. Moreover, BEX2 crotonylation at K59 is critical in the BEX2-mediated mitophagy in lung cancer cells. The K59R mutation of BEX2 inhibits mitophagy by affecting the interaction of NDP52 and LC3B. BEX2 expression is elevated after anticancer drug treatment, and its overexpression inhibits chemotherapy-induced apoptosis. In addition, inhibition of BEX2-regulated mitophagy sensitizes tumor cells to apoptosis. Furthermore, BEX2 promotes tumor growth and inhibits apoptosis by regulating mitophagy in vivo. We also confirm that BEX2 is overexpressed in lung adenocarcinoma and is associated with poor prognosis in lymph node metastasis-free cancer. Therefore, combination treatment with pharmaceutical approaches targeting BEX2-induced mitophagy and anticancer drugs may represent a potential strategy for NSCLC therapy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Naderi A, Teschendorff AE, Beigel J, Cariati M, Ellis IO, Brenton JD, et al. BEX2 is overexpressed in a subset of primary breast cancers and mediates nerve growth factor/nuclear factor-kappaB inhibition of apoptosis in breast cancer cell lines. Cancer Res. 2007;67:6725–36. doi: 10.1158/0008-5472.CAN-06-4394. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

