Effects of DJK-5 and chlorhexidine on exopolysaccharide volume and pH in oral biofilms
- PMID: 37777729
- PMCID: PMC10544135
- DOI: 10.1186/s12903-023-03381-5
Effects of DJK-5 and chlorhexidine on exopolysaccharide volume and pH in oral biofilms
Abstract
Background: Exopolysaccharides (EPS) are essential constituents of the extracellular matrix within oral biofilms and are significantly influenced by the local microenvironment. This study aimed to investigate the impact of two distinct antimicrobial agents, DJK-5 and chlorhexidine (CHX), on the EPS volume and pH levels in oral biofilms.
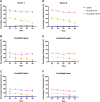
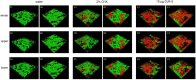
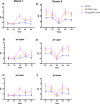
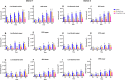
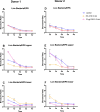
Methods: Oral biofilms obtained from two donors were cultured on hydroxyapatite discs for durations of 3 days, 1 week, 2 weeks, 3 weeks, and 4 weeks. Subsequently, these biofilms were subjected to treatment with 10 µg/mL DJK-5 or 2% CHX for 3 min. The impact of these antimicrobial treatments on factors such as the proportion of dead bacterial, in situ pH, and EPS volume within the biofilms was assessed using corresponding fluorescent probes. The examination was carried out utilizing confocal laser scanning microscopy, and the resulting images were analyzed with a focus on the upper and lower layers of the biofilm, respectively.
Results: DJK-5 exhibited a more potent bactericidal effect compared to CHX across the 3-day to 4-week duration of the biofilm (P < 0.05). The biofilms were acidic, with the upper layer being less acidic than the lower layer (P < 0.05). Both antimicrobial agents increased the pH, but DJK-5 had a greater effect than CHX (P < 0.05). The volume of EPS was significantly lower in DJK-5 treated biofilms compared to that of CHX, regardless of age or layer (P < 0.05).
Conclusion: DJK-5 exhibited superior effectiveness in reducing viable bacteria and EPS volume, as well as in raising extracellular pH, as compared to chlorhexidine.
Keywords: Chlorhexidine; Confocal laser scanning microscope; DJK-5; Exopolysaccharides; Oral biofilms; pH.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Recovery of Oral In Vitro Biofilms after Exposure to Peptides and Chlorhexidine.J Endod. 2021 Mar;47(3):466-471. doi: 10.1016/j.joen.2020.11.020. Epub 2020 Nov 25. J Endod. 2021. PMID: 33248060
-
Antibiofilm Effect of D-enantiomeric Peptide Alone and Combined with EDTA In Vitro.J Endod. 2017 Nov;43(11):1862-1867. doi: 10.1016/j.joen.2017.06.037. Epub 2017 Sep 23. J Endod. 2017. PMID: 28951034
-
Effect of Long-term Exposure to Peptides on Mono- and Multispecies Biofilms in Dentinal Tubules.J Endod. 2019 Dec;45(12):1522-1528. doi: 10.1016/j.joen.2019.09.003. Epub 2019 Oct 9. J Endod. 2019. PMID: 31606145
-
Characterization and application of a flow system for in vitro multispecies oral biofilm formation.J Periodontal Res. 2014 Jun;49(3):323-32. doi: 10.1111/jre.12110. Epub 2013 Jul 1. J Periodontal Res. 2014. PMID: 23815431
-
Effect of pomegranate solution alone or combined with chlorhexidine against oral multispecies biofilm.Int Endod J. 2024 Dec;57(12):1819-1828. doi: 10.1111/iej.14135. Epub 2024 Aug 10. Int Endod J. 2024. PMID: 39126293
Cited by
-
Endodontic Retreatment of a Mandibular Second Molar With a C-shaped Root Canal Configuration: A Case Report.Cureus. 2024 Jan 23;16(1):e52812. doi: 10.7759/cureus.52812. eCollection 2024 Jan. Cureus. 2024. PMID: 38389597 Free PMC article.
-
Locating and Treating Three Calcified Canals in a Mandibular First Molar With Radix Entomolaris and Five Canals: A Case Report.Cureus. 2024 Jan 25;16(1):e52931. doi: 10.7759/cureus.52931. eCollection 2024 Jan. Cureus. 2024. PMID: 38406086 Free PMC article.
-
Constructing two bifunctional tooth-targeting antimicrobial peptides for caries management: an in vitro study.Clin Oral Investig. 2024 Dec 31;29(1):36. doi: 10.1007/s00784-024-06139-7. Clin Oral Investig. 2024. PMID: 39739049
-
The Influence of Nickel-Titanium (Ni-Ti) Rotary Instrument Systems on Debris and Smear Layer Formation in Endodontic Procedures: An In Vitro Scanning Electron Microscopy Study.Cureus. 2024 Feb 16;16(2):e54310. doi: 10.7759/cureus.54310. eCollection 2024 Feb. Cureus. 2024. PMID: 38496119 Free PMC article.
-
Endodontic Treatment of a Maxillary Second Molar With Five Canals: A Case Report and a Literature Review.Cureus. 2024 Apr 27;16(4):e59179. doi: 10.7759/cureus.59179. eCollection 2024 Apr. Cureus. 2024. PMID: 38807838 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

