Oxidative stress as a key modulator of cell fate decision in osteoarthritis and osteoporosis: a narrative review
- PMID: 37777764
- PMCID: PMC10541721
- DOI: 10.1186/s11658-023-00489-y
Oxidative stress as a key modulator of cell fate decision in osteoarthritis and osteoporosis: a narrative review
Abstract
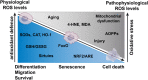
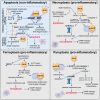
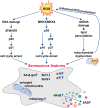
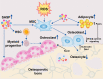
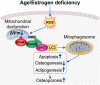
During aging and after traumatic injuries, cartilage and bone cells are exposed to various pathophysiologic mediators, including reactive oxygen species (ROS), damage-associated molecular patterns, and proinflammatory cytokines. This detrimental environment triggers cellular stress and subsequent dysfunction, which not only contributes to the development of associated diseases, that is, osteoporosis and osteoarthritis, but also impairs regenerative processes. To counter ROS-mediated stress and reduce the overall tissue damage, cells possess diverse defense mechanisms. However, cellular antioxidative capacities are limited and thus ROS accumulation can lead to aberrant cell fate decisions, which have adverse effects on cartilage and bone homeostasis. In this narrative review, we address oxidative stress as a major driver of pathophysiologic processes in cartilage and bone, including senescence, misdirected differentiation, cell death, mitochondrial dysfunction, and impaired mitophagy by illustrating the consequences on tissue homeostasis and regeneration. Moreover, we elaborate cellular defense mechanisms, with a particular focus on oxidative stress response and mitophagy, and briefly discuss respective therapeutic strategies to improve cell and tissue protection.
Keywords: Bone; Cartilage; Cell death; Cell fate decision; Mitochondrial dysfunction; Osteoarthritis; Osteoporosis; Oxidative stress; ROS; Senescence.
© 2023. University of Wroclav.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

