Inhibition of neutrophil extracellular trap formation ameliorates neuroinflammation and neuronal apoptosis via STING-dependent IRE1α/ASK1/JNK signaling pathway in mice with traumatic brain injury
- PMID: 37777772
- PMCID: PMC10543875
- DOI: 10.1186/s12974-023-02903-w
Inhibition of neutrophil extracellular trap formation ameliorates neuroinflammation and neuronal apoptosis via STING-dependent IRE1α/ASK1/JNK signaling pathway in mice with traumatic brain injury
Abstract
Background: Neuroinflammation is one of the most important pathogeneses in secondary brain injury after traumatic brain injury (TBI). Neutrophil extracellular traps (NETs) forming neutrophils were found throughout the brain tissue of TBI patients and elevated plasma NET biomarkers correlated with worse outcomes. However, the biological function and underlying mechanisms of NETs in TBI-induced neural damage are not yet fully understood. Here, we used Cl-amidine, a selective inhibitor of NETs to investigate the role of NETs in neural damage after TBI.
Methods: Controlled cortical impact model was performed to establish TBI. Cl-amidine, 2'3'-cGAMP (an activator of stimulating Interferon genes (STING)), C-176 (a selective STING inhibitor), and Kira6 [a selectively phosphorylated inositol-requiring enzyme-1 alpha [IRE1α] inhibitor] were administrated to explore the mechanism by which NETs promote neuroinflammation and neuronal apoptosis after TBI. Peptidyl arginine deiminase 4 (PAD4), an essential enzyme for neutrophil extracellular trap formation, is overexpressed with adenoviruses in the cortex of mice 1 day before TBI. The short-term neurobehavior tests, magnetic resonance imaging (MRI), laser speckle contrast imaging (LSCI), Evans blue extravasation assay, Fluoro-Jade C (FJC), TUNEL, immunofluorescence, enzyme-linked immunosorbent assay (ELISA), western blotting, and quantitative-PCR were performed in this study.
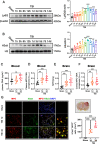
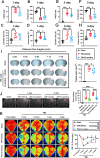
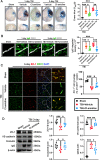
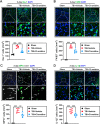
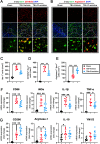
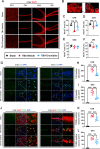
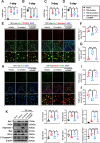
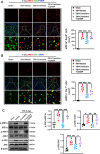
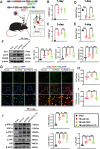
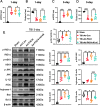
Results: Neutrophils form NETs presenting in the circulation and brain at 3 days after TBI. NETs inhibitor Cl-amidine treatment improved short-term neurological functions, reduced cerebral lesion volume, reduced brain edema, and restored cerebral blood flow (CBF) after TBI. In addition, Cl-amidine exerted neuroprotective effects by attenuating BBB disruption, inhibiting immune cell infiltration, and alleviating neuronal death after TBI. Moreover, Cl-amidine treatment inhibited microglia/macrophage pro-inflammatory polarization and promoted anti-inflammatory polarization at 3 days after TBI. Mechanistically, STING ligand 2'3'-cGAMP abolished the neuroprotection of Cl-amidine via IRE1α/ASK1/JNK signaling pathway after TBI. Importantly, overexpression of PAD4 promotes neuroinflammation and neuronal death via the IRE1α/ASK1/JNK signaling pathway after TBI. However, STING inhibitor C-176 or IRE1α inhibitor Kira6 effectively abolished the neurodestructive effects of PAD4 overexpression after TBI.
Conclusion: Altogether, we are the first to demonstrate that NETs inhibition with Cl-amidine ameliorated neuroinflammation, neuronal apoptosis, and neurological deficits via STING-dependent IRE1α/ASK1/JNK signaling pathway after TBI. Thus, Cl-amidine treatment may provide a promising therapeutic approach for the early management of TBI.
Keywords: Cl-amidine; IRE1α; Neuroinflammation; Neuronal apoptosis; Neutrophil extracellular traps; STING; Traumatic brain injury.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Tehse J, Taghibiglou C. The overlooked aspect of excitotoxicity: glutamate-independent excitotoxicity in traumatic brain injuries. Eur J Neurosci. 2019;49(9):1157–1170. - PubMed
-
- Morganti-Kossmann MC, et al. The complexity of neuroinflammation consequent to traumatic brain injury: from research evidence to potential treatments. Acta Neuropathol. 2019;137(5):731–755. - PubMed
-
- Willis EF, et al. Repopulating microglia promote brain repair in an IL-6-dependent manner. Cell. 2020;180(5):833–846.e16. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

