Spatial proteomics of hippocampal subfield-specific pathology in Alzheimer's disease and primary age-related tauopathy
- PMID: 37777848
- PMCID: PMC10916977
- DOI: 10.1002/alz.13484
Spatial proteomics of hippocampal subfield-specific pathology in Alzheimer's disease and primary age-related tauopathy
Abstract
Introduction: Alzheimer's disease (AD) and primary age-related tauopathy (PART) both harbor 3R/4R hyperphosphorylated-tau (p-tau)-positive neurofibrillary tangles (NFTs) but differ in the spatial p-tau development in the hippocampus.
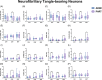
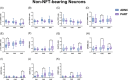
Methods: Using Nanostring GeoMx Digital Spatial Profiling, we compared protein expression within hippocampal subregions in NFT-bearing and non-NFT-bearing neurons in AD (n = 7) and PART (n = 7) subjects.
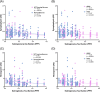
Results: Proteomic measures of synaptic health were inversely correlated with the subregional p-tau burden in AD and PART, and there were numerous differences in proteins involved in proteostasis, amyloid beta (Aβ) processing, inflammation, microglia, oxidative stress, and neuronal/synaptic health between AD and PART and between definite PART and possible PART.
Discussion: These results suggest subfield-specific proteome differences that may explain some of the differences in Aβ and p-tau distribution and apparent pathogenicity. In addition, hippocampal neurons in possible PART may have more in common with AD than with definite PART, highlighting the importance of Aβ in the pathologic process.
Highlights: Synaptic health is inversely correlated with local p-tau burden. The proteome of NFT- and non-NFT-bearing neurons is influenced by the presence of Aβ in the hippocampus. Neurons in possible PART cases share more proteomic similarities with neurons in ADNC than they do with neurons in definite PART cases.
Keywords: Alzheimer's disease (AD); aging; neurodegeneration; primary age-related tauopathy (PART); resilience; resistance; synapses.
© 2023 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Preliminary results of the data presented in this paper were published in abstract form for the 2023 American Association of Neuropathologists and 2023 Alzheimer's Association International Conference. The authors declare that they have no competing interests, conflicts of interest, or other relevant disclosures. Author disclosures are available in the supporting information.
Figures




References
-
- Thal DR, Rub U, Orantes M, Braak H. Phases of A beta‐deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791‐1800. - PubMed
-
- Braak H, Braak E. Neuropathological stageing of Alzheimer‐related changes. Acta Neuropathol. 1991;82:239‐259. - PubMed
-
- Jellinger KA. Different patterns of hippocampal tau pathology in Alzheimer's disease and PART. Acta Neuropathol. 2018;136:811‐813. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG065839/AG/NIA NIH HHS/United States
- P30 AG066512/AG/NIA NIH HHS/United States
- T32 AG049688/AG/NIA NIH HHS/United States
- R21 AG078505/AG/NIA NIH HHS/United States
- R21 NS125171/NS/NINDS NIH HHS/United States
- U54 AG079754/AG/NIA NIH HHS/United States
- P01 AG060882/AG/NIA NIH HHS/United States
- K01 AG070326/AG/NIA NIH HHS/United States
- R01 AG068293/AG/NIA NIH HHS/United States
- P30 AG066546/AG/NIA NIH HHS/United States
- R24 AG073199/AG/NIA NIH HHS/United States
- P30 AG044271/AG/NIA NIH HHS/United States
- P30 AG066514/AG/NIA NIH HHS/United States
- I01 BX005717/BX/BLRD VA/United States
LinkOut - more resources
Full Text Sources
Medical

