Citrullination of matrisomal proteins in health and diseases
- PMID: 37778384
- PMCID: PMC10542447
- DOI: 10.1098/rstb.2022.0244
Citrullination of matrisomal proteins in health and diseases
Abstract
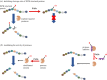
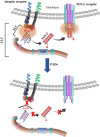
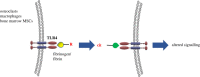
Proteins once translated are subjected to post-translational modifications (PTMs) that can critically modify their characteristics. Citrullination is a unique type of PTM that is catalysed by peptidylarginine deiminase (PAD) enzymes, which regulate a multitude of physiological functions such as apoptosis, gene expression and immune response by altering the structure and function of cellular proteins. However, emerging data have unravelled compelling evidence to support that PAD-mediated citrullination is not exclusive to cellular proteins; rather citrullination of extracellular matrix (ECM) proteins also plays a major contributing role in various physiological/pathological conditions. Here, we discuss putative mechanisms for citrullination-induced alterations in the function of ECM proteins. Further, we put emphasis on influential roles of ECM citrullination in various pathological scenarios to underscore the clinical potential of its manipulation in human diseases. This article is part of the Theo Murphy meeting issue 'The virtues and vices of protein citrullination'.
Keywords: citrullination; deiminase; deimination; extracellular matrix; post-translational modification.
Conflict of interest statement
We declare we have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
