Belatacept with time-limited tacrolimus coimmunosuppression modifies the 3-year risk of eplet mismatch in kidney transplantation
- PMID: 37778459
- PMCID: PMC10842047
- DOI: 10.1016/j.ajt.2023.09.011
Belatacept with time-limited tacrolimus coimmunosuppression modifies the 3-year risk of eplet mismatch in kidney transplantation
Abstract
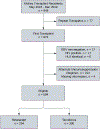
Solid organ transplant donor-recipient eplet mismatch has been correlated with donor-specific antibody (DSA) formation, antibody-mediated rejection, and overall rejection rates. However, studies have been predominantly in patients on tacrolimus-based immunosuppression regimens and have not fully explored differences in ethnically and racially diverse populations. Evidence indicates that patients on belatacept have lower rates of DSA formation, suggesting mediation of the immunogenicity of mismatched human leukocyte antigen polymorphisms. We performed a retrospective, single-center analysis of class II eplet disparity in a cohort of kidney transplant recipients treated using belatacept with tacrolimus induction (Bela/TacTL) or tacrolimus regimens between 2016 and 2019. Bela/TacTL (n = 294) and tacrolimus (n = 294) cohorts were propensity score-matched with standardized difference <0.15. Single-molecule eplet risk level was associated with immune event rates for both groups. In Cox regression analysis stratified by eplet risk level, Bela/TacTL immunosuppression was associated with a decreased rate of DSA (hazard ratio [HR] = 0.4), antibody-mediated rejection (HR = 0.2), and rejection (HR = 0.45). In the low-risk group, cumulative graft failure was lower for patients on Bela/TacTL (P < .02). Analysis of eplet mismatch burden may be a useful adjunct in identifying high-risk populations with increased immunosuppression requirements and should encourage the design of allocation rules to incentivize lower-risk pairings without negatively impacting equity in access.
Keywords: HLA; antibody; belatacept; costimulation; eplet; immunosuppression; rejection.
Copyright © 2023 American Society of Transplantation & American Society of Transplant Surgeons. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
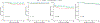
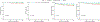


Similar articles
-
Eplet Mismatch Load and De Novo Occurrence of Donor-Specific Anti-HLA Antibodies, Rejection, and Graft Failure after Kidney Transplantation: An Observational Cohort Study.J Am Soc Nephrol. 2020 Sep;31(9):2193-2204. doi: 10.1681/ASN.2020010019. Epub 2020 Aug 6. J Am Soc Nephrol. 2020. PMID: 32764139 Free PMC article.
-
Eplet mismatch analysis and allograft outcome across racially diverse groups in a pediatric transplant cohort: a single-center analysis.Pediatr Nephrol. 2020 Jan;35(1):83-94. doi: 10.1007/s00467-019-04344-1. Epub 2019 Oct 10. Pediatr Nephrol. 2020. PMID: 31599339 Free PMC article.
-
Combined Analysis of HLA Class II Eplet Mismatch and Tacrolimus Levels for the Prediction of De Novo Donor Specific Antibody Development in Kidney Transplant Recipients.Int J Mol Sci. 2022 Jul 1;23(13):7357. doi: 10.3390/ijms23137357. Int J Mol Sci. 2022. PMID: 35806362 Free PMC article.
-
Preformed T cell alloimmunity and HLA eplet mismatch to guide immunosuppression minimization with tacrolimus monotherapy in kidney transplantation: Results of the CELLIMIN trial.Am J Transplant. 2021 Aug;21(8):2833-2845. doi: 10.1111/ajt.16563. Epub 2021 Apr 15. Am J Transplant. 2021. PMID: 33725408 Clinical Trial.
-
Human leukocyte antigen epitope mismatch loads and the development of de novo donor-specific antibodies in cardiothoracic organ transplantation.Int J Immunogenet. 2022 Feb;49(1):30-38. doi: 10.1111/iji.12563. Epub 2021 Dec 14. Int J Immunogenet. 2022. PMID: 34904369 Review.
Cited by
-
Immunosuppression Management in Heart Transplantation.Methodist Debakey Cardiovasc J. 2025 May 15;21(3):40-50. doi: 10.14797/mdcvj.1596. eCollection 2025. Methodist Debakey Cardiovasc J. 2025. PMID: 40384742 Free PMC article. Review.
-
Implications of MHC-restricted immunopeptidome in transplantation.Front Immunol. 2024 Jul 5;15:1436233. doi: 10.3389/fimmu.2024.1436233. eCollection 2024. Front Immunol. 2024. PMID: 39035001 Free PMC article. Review.
-
HLA-DR/DQ eplet mismatch predicts de novo donor-specific antibody development in multi-ethnic Southeast Asian kidney transplant recipients on different immunosuppression regimens.Front Genet. 2024 Aug 28;15:1447141. doi: 10.3389/fgene.2024.1447141. eCollection 2024. Front Genet. 2024. PMID: 39262421 Free PMC article.
-
Molecular matching tools for allocation and immunosuppression optimization. Ready for primetime?Curr Opin Organ Transplant. 2025 Feb 1;30(1):30-36. doi: 10.1097/MOT.0000000000001185. Epub 2024 Nov 12. Curr Opin Organ Transplant. 2025. PMID: 39711242 Free PMC article. Review.
-
Belatacept in Kidney Transplantation: Reflecting on the Past, Shaping the Future.Transpl Int. 2025 May 20;38:14412. doi: 10.3389/ti.2025.14412. eCollection 2025. Transpl Int. 2025. PMID: 40463417 Free PMC article. Review.
References
-
- Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR). OPTN/SRTR 2020 Annual Data Report. Published 2022. Accessed April 4, 2022.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

