Regulatory T cells in dominant immunologic tolerance
- PMID: 37778472
- PMCID: PMC10842646
- DOI: 10.1016/j.jaci.2023.09.025
Regulatory T cells in dominant immunologic tolerance
Abstract
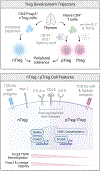
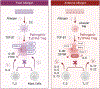
Regulatory T cells expressing the transcription factor forkhead box protein 3 mediate peripheral immune tolerance both to self-antigens and to the commensal flora. Their defective function due to inborn errors of immunity or acquired insults is associated with a broad range of autoimmune and immune dysregulatory diseases. Although their function in suppressing autoimmunity and enforcing commensalism is established, a broader role for regulatory T cells in tissue repair and metabolic regulation has emerged, enabled by unique programs of tissue adaptability and specialization. In this review, we focus on the myriad roles played by regulatory T cells in immunologic tolerance and host homeostasis and the potential to harness these cells in novel therapeutic approaches to human diseases.
Keywords: Foxp3; IL-2; Regulatory T cells; autoimmunity; immunologic tolerance; regulatory T-cell therapy.
Copyright © 2023 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests
S.H. has consulted for Merck KGaA. P.G. has consulted for RA Capital and Astro Therapeutics. A.H.S. currently has funding from Quark and AbbVie, unrelated to the submitted work. A.H.S. serves on advisory boards for SQZ Biotechnologies, Selecta, Elpiscience, Monopteros, Bicara, Fibrogen, IOME, Corner Therapeutics and Alixia. She also is on scientific advisory boards for the Massachusetts General Cancer Center, Program in Cellular and Molecular Medicine at Boston Children’s Hospital, the Human Oncology and Pathogenesis Program at Memorial Sloan Kettering Cancer Center, Bloomberg-Kimmel Institute for Cancer Immunotherapy, Glaxo Smith Kline, Janssen and Amgen. She is an academic editor for the Journal of Experimental Medicine. A.H.S. has patents/pending royalties on the PD-1 pathway from Roche and Novartis. M.C.H. has patents pending on the PHD3 pathway, is on the scientific advisory board for Alixia, Minovia, and MitoQ, and had research funding from Roche.
Figures
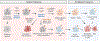



References
-
- Silverstein AM. Autoimmunity versus horror autotoxicus: the struggle for recognition. Nat Immunol 2001; 2:279–81. - PubMed
-
- Brent L. The discovery of immunologic tolerance. Hum Immunol 1997; 52:75–81. - PubMed
-
- Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell 1987; 49:273–80. - PubMed
-
- Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature 1988; 333:742–6. - PubMed
-
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science 2002; 298:1395–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

