Immunogenicity of lipid nanoparticles and its impact on the efficacy of mRNA vaccines and therapeutics
- PMID: 37779140
- PMCID: PMC10618257
- DOI: 10.1038/s12276-023-01086-x
Immunogenicity of lipid nanoparticles and its impact on the efficacy of mRNA vaccines and therapeutics
Abstract
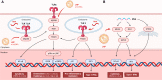
Several studies have utilized a lipid nanoparticle delivery system to enhance the effectiveness of mRNA therapeutics and vaccines. However, these nanoparticles are recognized as foreign materials by the body and stimulate innate immunity, which in turn impacts adaptive immunity. Therefore, it is crucial to understand the specific type of innate immune response triggered by lipid nanoparticles. This article provides an overview of the immunological response in the body, explores how lipid nanoparticles activate the innate immune system, and examines the adverse effects and immunogenicity-related development pathways associated with these nanoparticles. Finally, we highlight and explore strategies for regulating the immunogenicity of lipid nanoparticles.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

