Activation of multiple Eph receptors on neuronal membranes correlates with the onset of optic neuropathy
- PMID: 37779186
- PMCID: PMC10544557
- DOI: 10.1186/s40662-023-00359-w
Activation of multiple Eph receptors on neuronal membranes correlates with the onset of optic neuropathy
Abstract
Background: Optic neuropathy is a major cause of irreversible blindness, yet the molecular determinants that contribute to neuronal demise have not been fully elucidated. Several studies have identified 'ephrin signaling' as one of the most dysregulated pathways in the early pathophysiology of optic neuropathy with varied etiologies. Developmentally, gradients in ephrin signaling coordinate retinotopic mapping via repulsive modulation of cytoskeletal dynamics in neuronal membranes. Little is known about the role ephrin signaling plays in the post-natal visual system and its correlation with the onset of optic neuropathy.
Methods: Postnatal mouse retinas were collected for mass spectrometry analysis for erythropoietin-producing human hepatocellular (Eph) receptors. Optic nerve crush (ONC) model was employed to induce optic neuropathy, and proteomic changes during the acute phase of neuropathic onset were analyzed. Confocal and super-resolution microscopy determined the cellular localization of activated Eph receptors after ONC injury. Eph receptor inhibitors assessed the neuroprotective effect of ephrin signaling modulation.
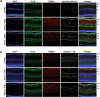
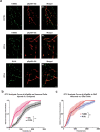
Results: Mass spectrometry revealed expression of seven Eph receptors (EphA2, A4, A5, B1, B2, B3, and B6) in postnatal mouse retinal tissue. Immunoblotting analysis indicated a significant increase in phosphorylation of these Eph receptors 48 h after ONC. Confocal microscopy demonstrated the presence of both subclasses of Eph receptors within the retina. Stochastic optical reconstruction microscopy (STORM) super-resolution imaging combined with optimal transport colocalization analysis revealed a significant co-localization of activated Eph receptors with injured neuronal cells, compared to uninjured neuronal and/or injured glial cells, 48 h post-ONC. Eph receptor inhibitors displayed notable neuroprotective effects for retinal ganglion cells (RGCs) after six days of ONC injury.
Conclusions: Our findings demonstrate the functional presence of diverse Eph receptors in the postnatal mammalian retina, capable of modulating multiple biological processes. Pan-Eph receptor activation contributes to the onset of neuropathy in optic neuropathies, with preferential activation of Eph receptors on neuronal processes in the inner retina following optic nerve injury. Notably, Eph receptor activation precedes neuronal loss. We observed a neuroprotective effect on RGCs upon inhibiting Eph receptors. Our study highlights the importance of investigating this repulsive pathway in early optic neuropathies and provides a comprehensive characterization of the receptors present in the developed retina of mice, relevant to both homeostasis and disease processes.
Keywords: Axonal guidance; Eph receptor; Ephrin signaling; Neurodegeneration; Neuropathy; Neuroprotection; Optic nerve; Optic nerve crush; Optic neuropathy; Retinal ganglion cell.
© 2023. Wenzhou Medical University.
Conflict of interest statement
Dr. Pelaez is a consultant and equity holder in EIR Biopharma. The other authors have no actual or perceived competing interests to declare.
Figures






Update of
-
Activation of Multiple Eph Receptors on Neuronal Membranes Correlates with The Onset of Traumatic Optic Neuropathy.bioRxiv [Preprint]. 2023 Jun 7:2023.06.05.543735. doi: 10.1101/2023.06.05.543735. bioRxiv. 2023. Update in: Eye Vis (Lond). 2023 Oct 2;10(1):42. doi: 10.1186/s40662-023-00359-w. PMID: 37333178 Free PMC article. Updated. Preprint.
Similar articles
-
Activation of Multiple Eph Receptors on Neuronal Membranes Correlates with The Onset of Traumatic Optic Neuropathy.bioRxiv [Preprint]. 2023 Jun 7:2023.06.05.543735. doi: 10.1101/2023.06.05.543735. bioRxiv. 2023. Update in: Eye Vis (Lond). 2023 Oct 2;10(1):42. doi: 10.1186/s40662-023-00359-w. PMID: 37333178 Free PMC article. Updated. Preprint.
-
Gradients of Eph-A6 expression in primate retina suggest roles in both vascular and axon guidance.Mol Vis. 2009 Dec 9;15:2649-62. Mol Vis. 2009. PMID: 20011078 Free PMC article.
-
BAX-Depleted Retinal Ganglion Cells Survive and Become Quiescent Following Optic Nerve Damage.Mol Neurobiol. 2020 Feb;57(2):1070-1084. doi: 10.1007/s12035-019-01783-7. Epub 2019 Oct 31. Mol Neurobiol. 2020. PMID: 31673950 Free PMC article.
-
Neuroinflammation, Microglia and Implications for Retinal Ganglion Cell Survival and Axon Regeneration in Traumatic Optic Neuropathy.Front Immunol. 2022 Mar 4;13:860070. doi: 10.3389/fimmu.2022.860070. eCollection 2022. Front Immunol. 2022. PMID: 35309305 Free PMC article. Review.
-
Roles of Eph/ephrin bidirectional signaling during injury and recovery of the central nervous system.Neural Regen Res. 2018 Aug;13(8):1313-1321. doi: 10.4103/1673-5374.235217. Neural Regen Res. 2018. Retraction in: Neural Regen Res. 2018 Dec;13(12):2140. doi: 10.4103/1673-5374.242462. PMID: 30106032 Free PMC article. Retracted. Review.
Cited by
-
Repurposing development genes for axonal regeneration following injury: Examining the roles of Wnt signaling.Front Cell Dev Biol. 2024 May 31;12:1417928. doi: 10.3389/fcell.2024.1417928. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38882059 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

