C500 variants conveying complete mucosal immunity against fatal infections of pigs with Salmonella enterica serovar Choleraesuis C78-1 or F18+ Shiga toxin-producing Escherichia coli
- PMID: 37779705
- PMCID: PMC10536267
- DOI: 10.3389/fmicb.2023.1210358
C500 variants conveying complete mucosal immunity against fatal infections of pigs with Salmonella enterica serovar Choleraesuis C78-1 or F18+ Shiga toxin-producing Escherichia coli
Abstract
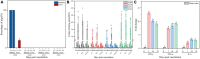
Salmonella enterica serovar Choleraesuis (S. Choleraesuis) C500 strain is a live, attenuated vaccine strain that has been used in China for over 40 years to prevent piglet paratyphoid. However, this vaccine is limited by its toxicity and does not offer protection against diseases caused by F18+ Shiga toxin-producing Escherichia coli (STEC), which accounts for substantial economic losses in the swine industry. We recently generated a less toxic derivative of C500 strain with both asd and crp deletion (S. Choleraesuis C520) and assessed its efficacy in mice. In addition, we demonstrate that C520 is also less toxic in pigs and is effective in protecting pigs against S. Choleraesuis when administered orally. To develop a vaccine with a broader range of protection, we prepared a variant of C520 (S. Choleraesuis C522), which expresses rSF, a fusion protein comprised of the fimbriae adhesin domain FedF and the Shiga toxin-producing IIe B domain antigen. For comparison, we also prepared a control vector strain (S. Choleraesuis C521). After oral vaccination of pigs, these strains contributed to persistent colonization of the intestinal mucosa and lymphoid tissues and elicited both cytokine expression and humoral immune responses. Furthermore, oral immunization with C522 elicited both S. Choleraesuis and rSF-specific immunoglobulin G (IgG) and IgA antibodies in the sera and gut mucosa, respectively. To further evaluate the feasibility and efficacy of these strains as mucosal delivery vectors via oral vaccination, we evaluated their protective efficacy against fatal infection with S. Choleraesuis C78-1, as well as the F18+ Shiga toxin-producing Escherichia coli field strain Ee, which elicits acute edema disease. C521 conferred complete protection against fatal infection with C78-1; and C522 conferred complete protection against fatal infection with both C78-1 and Ee. Our results suggest that C520, C521, and C522 are competent to provide complete mucosal immune protection against fatal infection with S. Choleraesuis in swine and that C522 equally qualifies as an oral vaccine vector for protection against F18+ Shiga toxin-producing Escherichia coli.
Keywords: Salmonella enterica serovar Choleraesuis C500; edema disease of swine; host; in vivo; mucosal immunity.
Copyright © 2023 Liu, Li, Liao, Guo, Wu and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Alborali G. L., Ruggeri J., Pesciaroli M., Martinelli N., Chirullo B., Ammendola S., et al. (2017). Prime-boost vaccination with attenuated Salmonella typhimurium ΔznuABC and inactivated Salmonella choleraesuis is protective against Salmonella choleraesuis challenge infection in piglets. BMC Vet. Res. 13:284. 10.1186/s12917-017-1202-5 - DOI - PMC - PubMed
-
- Atul A. C., Sam Woong K., Kiku M., John Hwa L. (2011). Safety evaluation and immunogenicity of arabinose-based conditional lethal Salmonella Gallinarum mutant unable to survive ex vivo as a vaccine candidate for protection against fowl typhoid. Avian Dis. 55 165–171. 10.1637/9512-083010-Reg.1 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

