The anaemia treatment journey of CKD patients: from epoetins to hypoxia-inducible factor-prolyl hydroxylase inhibitors
- PMID: 37779852
- PMCID: PMC10539216
- DOI: 10.1093/ckj/sfad105
The anaemia treatment journey of CKD patients: from epoetins to hypoxia-inducible factor-prolyl hydroxylase inhibitors
Abstract
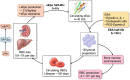
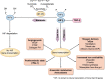
The discovery and development of erythropoiesis-stimulating agents was a journey lasting more than a century, leading to the cloning and approval of recombinant human erythropoietin (rHuEpo). This was an impressive clinical advance, providing the possibility of correcting the symptoms associated with anaemia in chronic kidney disease. Associated iron use was needed to produce new haemoglobin-containing blood red cells. Partial anaemia correction became the standard of care since trials aiming for near-normal haemoglobin levels showed a higher risk of adverse cardiovascular events. Hoping to reduce the cardiovascular risks, a new category of drugs was developed and tested. Hypoxia-inducible factor prolyl hydroxylase inhibitors (HIF-PHIs) are small molecules than can be formulated into orally active pills. They simulate reduced tissue oxygen pressure, thus stimulating the production of endogenous erythropoietin (Epo) by the kidneys and liver. Clinical trials with these compounds demonstrated that HIF-PHIs are at least as effective as rHuEpo in treating or correcting anaemia in non-dialysis and dialysis patients. Trials with HIF-PHIs did not demonstrate superiority in safety outcomes and in some trials, outcomes were worse. There was also a focus on oral delivery, a possible beneficial iron-sparing effect and the ability to overcome Epo resistance in inflamed patients. A negative effect is possible iron depletion, which may explain adverse outcomes.
Keywords: adherence; anaemia; chronic kidney disease; dialysis; erythropoiesis-stimulating agents.
© The Author(s) 2023. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
F.L. is or was a member of an advisory board for Amgen, Astellas, Baxter, GlaxoSmithKline, Otsuka, Travere and Vifor Pharma and was a speaker at meetings supported by Amgen, Astellas and Vifor Pharma. L.D.V. has participated in advisory boards for Astellas, GlaxoSmithKline and Travere. She received speaker fees for a meeting indirectly supported by Vifor Pharma and Amgen, Astellas. S.E. is a former employee, consultant and current stockholder of Amgen and was a consultant but currently receives no financial compensation from Amgen. He is an inventor of ESA-related patents, but is not the assignee, and receives no personal financial benefit from them.
Figures

References
-
- Jourdanet D. De l'anemie des altitudes et de l'anemie en general dans ses rapports avec la pression del l'atmosphere. Paris: Balliere, 1863.
-
- Miyake T, Kung CK, Goldwasser E. Purification of human erythropoietin. J Biol Chem 1977;252:5558–64. - PubMed
-
- Eschbach JW, Egrie JC, Downing MRet al. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med 1987;316:73–8. - PubMed
-
- Winearls CG, Forman E, Wiffen Pet al. Recombinant human erythropoietin treatment in patients on maintenance home haemodialysis. Lancet 1989;334:569. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

