Dps Functions as a Key Player in Bacterial Iron Homeostasis
- PMID: 37779979
- PMCID: PMC10536872
- DOI: 10.1021/acsomega.3c03277
Dps Functions as a Key Player in Bacterial Iron Homeostasis
Abstract
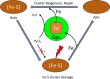
Iron plays a vital role in the maintenance of life, being central to various cellular processes, from respiration to gene regulation. It is essential for iron to be stored in a nontoxic and readily available form. DNA binding proteins under starvation (Dps) belong to the ferritin family of iron storage proteins and are adept at storing iron in their hollow protein shells. Existing solely in prokaryotes, these proteins have the additional functions of DNA binding and protection from oxidative stress. Iron storage proteins play a functional role in storage, release, and transfer of iron and therefore are central to the optimal functioning of iron homeostasis. Here we review the multifarious properties of Dps through relevant biochemical and structural studies with a focus on iron storage and ferroxidation. We also examine the role of Dps as a possible candidate as an iron donor to iron-sulfur (Fe-S) clusters, which are ubiquitous to many biological processes.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

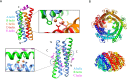

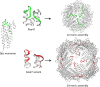
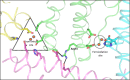
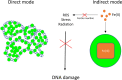
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

