Severe efavirenz associated neurotoxicity: A retrospective cohort study
- PMID: 37780199
- PMCID: PMC10397354
- DOI: 10.4102/sajid.v38i1.522
Severe efavirenz associated neurotoxicity: A retrospective cohort study
Abstract
Background: Efavirenz (EFV) is associated with neuropsychiatric symptoms. Severe neurotoxicity has been reported but the clinical phenotype and risk factors are poorly defined.
Objectives: To characterise clinical presentations, risk factors and outcomes to help clinicians recognise severe neurotoxicity earlier.
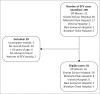
Method: The authors retrospectively identified adults with supratherapeutic EFV concentrations (> 4 mg/L) obtained during routine clinical care in Cape Town, South Africa. Clinical and laboratory data at the time of EFV quantification were extracted from medical records. Logistic regression was performed to identify associations with neuropsychiatric symptoms, and with severe neurotoxicity.
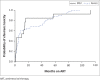
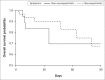
Results: Eighty one patients were included; 62 with neuropsychiatric manifestations (most frequently ataxia [n = 20] and psychomotor slowing [n = 24]); and 19 with hepatotoxicity. Overall, 28 (34.6%) were male, 49 (60.5%) had concomitant isoniazid exposure, and median EFV concentration was 12.1 mg/L (interquartile range [IQR]: 6.6-20.0). Neuropsychiatric symptoms were associated with longer duration of EFV therapy, adjusted odds ratio (aOR) 1.3/180-day increment (95% confidence interval [CI]: 1.0-1.7); higher EFV concentrations, aOR 1.2/1 mg/L increase (95% CI: 1.0-1.4) and isoniazid exposure, aOR 8.2 (95% CI: 2.5-26.7). Severe neuropsychiatric symptoms occurred in 47 (75%) patients at a median of 5.9 months (IQR: 2.1-40.8) after EFV initiation. Severe symptoms odds were 1.2-fold higher (95% CI: 1.1-1.4) per 1 mg/L increase in EFV concentration. Symptoms resolved completely within 1 month in 25 (76%) patients with severe neurotoxicity who discontinued EFV.
Conclusion: A concentration-effect relationship for severe neurotoxicity exists, which occurred late and resolved in most patients after EFV discontinuation.
Contribution: The authors highlighted clinical heterogeneity and morbidity of EFV-associated neurotoxicity.
Keywords: Cape Town; cerebellar; efavirenz; isoniazid; neurotoxicity; risk factors.
© 2023. The Authors.
Conflict of interest statement
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Figures

References
-
- HIV/AIDS JUNPo . UNAIDS South Africa. United Nations [homepage on the Internet]. 2018. [cited 2019 Oct 18]. Available from: http://www.unaids.org/en/regionscountries/countries/southafrica
-
- Masur H, Brooks JT, Benson CA, Holmes KK, Pau AK, Kaplan JE. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Updated guidelines from the Centres for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58(9):1308–1311. 10.1093/cid/ciu094 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
