Friend leukemia integration 1 overexpression decreases endometrial receptivity and induces embryo implantation failure by promoting PART1 transcription in the endometrial epithelial cells
- PMID: 37780395
- PMCID: PMC10540769
- DOI: 10.7717/peerj.16105
Friend leukemia integration 1 overexpression decreases endometrial receptivity and induces embryo implantation failure by promoting PART1 transcription in the endometrial epithelial cells
Abstract
Background: In vitro fertilization-embryo transfer (IVF-ET) is a crucial assisted reproductive technology for treating infertility. However, recurrent implantation failure (RIF), a significant challenge in IVF-ET success, remains unresolved. This study aimed to explore the role and mechanism of FLI1 in endometrial receptivity and RIF.
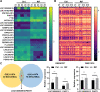
Methods: Differential endometrial cell proportions between patients with RIF and control subjects were assessed using single-cell RNA sequencing (scRNA-seq) analysis. The chromatin accessibility of FLI1 in the luteal endometrial tissue of patients with RIF and control subjects was examined using the single-cell assay for transposase-accessible chromatin sequencing (scATAC-seq). FLI1 mRNA and protein levels were gauged by quantitative real-time polymerase chain reaction (qRT-PCR) and western blotting. Cell viability and migration were examined via cell counting kit (CCK)-8 and scratch healing assays. Epithelial-mesenchymal transition markers were analyzed using western blotting. Mechanisms underlying FLI1's regulation of PART1 transcription and expression in endometrial epithelial cells were explored using chromatin immunoprecipitation and dual-luciferase reporter assays. Adeno-associated virus (AAV) carrying epithelial cell-specific FLI1/PART1 overexpression sequences was uterinely injected in mice to assess FLI1/PART1 effects.
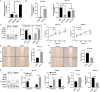
Results: scRNA-seq revealed diminished endometrial epithelial cell proportions in RIF patients. Meanwhile, scATAC-seq indicated enhanced chromatin accessibility of FLI1 in these cells. FLI1 exhibited specific expression in RIF patients' endometrial epithelial cells. Specific FLI1 overexpression inhibited embryo implantation, while knockdown enhanced it. Pregnant mice injected with AAV encoding FLI1 overexpression had significantly lower implantation than AAV-negative controls. FLI1 binding to PART1 promoter heightened PART1 transcription and expression in endometrial epithelial cells. Rescue experiments illustrated FLI1's role in embryo implantation by boosting PART1 expression. PART1 was notably elevated in RIF patients' luteal endometrial tissue and non-receptive endometrial epithelial cells (HEC-1-A). Specific PART1 overexpression dampened embryo implantation, whereas knockdown promoted it. Pregnant mice injected with AAV encoding PART1 had lower implantation than negative controls. PART1 knockdown mitigated FLI1's inhibitory impact on HEC-1-A cell viability and migration.
Conclusions: FLI1 overexpression in the endometrial epithelial cells of patients with RIF inhibited embryo implantation by binding to the PART1 promoter region to promote PART1 expression. These findings can aid in the development of novel therapeutic targets for RIF.
Keywords: Endometrial receptivity; FLI1; PART1; Recurrent implantation failure (RIF); Single cell chromatin accessibility sequencing (scATAC-seq).
©2023 Zhang et al.
Conflict of interest statement
The authors declare there are no competing interests.
Figures





References
-
- Corces MR, Trevino AE, Hamilton EG, Greenside PG, Sinnott-Armstrong NA, Vesuna S, Satpathy AT, Rubin AJ, Montine KS, Wu B, Kathiria A, Cho SW, Mumbach MR, Carter AC, Kasowski M, Orloff LA, Risca VI, Kundaje A, Khavari PA, Montine TJ, Greenleaf WJ, Chang HY. An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nature Methods. 2017;14:959–962. doi: 10.1038/nmeth.4396. - DOI - PMC - PubMed
-
- Crha K, Ventruba P, Žáková J, Ješeta M, Pilka R, Vodička J, Serpa P. The role of mesenchymal-epithelial transition in endometrial function and receptivity. Ceskoslovenska Gynekologie. 2019;84:371–375. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources

