MoS2-NiO nanocomposite for H2S sensing at room temperature
- PMID: 37780733
- PMCID: PMC10539850
- DOI: 10.1039/d3ra05241a
MoS2-NiO nanocomposite for H2S sensing at room temperature
Abstract
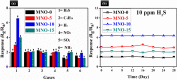
The layered 2-D materials, such as molybdenum disulfide (MoS2), are among the most promising candidates for detecting H2S gas at very low concentrations. Herein, we have designed a series of novel nanocomposites consisting of MoS2 and NiO. These materials were synthesized via a simple hydrothermal method. The microstructure and morphology of nanocomposites were studied using different characterization techniques, such as X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM), Brunauer-Emmett-Teller (BET) analysis, and X-ray photoelectron spectroscopy (XPS). These nanocomposites were used as gas sensors, and the highest response (6.3) towards 10 ppm H2S was detected by the MNO-10 gas sensor among all the tested sensors. The response value (Rg/Ra) was almost three times that of pure NiO (Rg/Ra = 2). Besides, the MNO-10 sensor exposed good selectivity, short response/recovery time (50/20 s), long-term stability (28 days), reproducibility (6 cycles), and a low detection limit (2 ppm) towards H2S gas at RT. The excellent performance of MNO-10 may be attributed to some features of MoS2, such as a layered structure, higher BET surface area, higher active sites, and a synergistic effect between MoS2 and NiO. This simple fabrication sensor throws a novel idea for detecting H2S gas.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
This work does not have any conflict of interest.
Figures










References
-
- Shirsat M. D. Bangar M. A. Deshusses M. A. Myung N. V. Mulchandani A. Polyaniline nanowires-gold nanoparticles hybrid network based chemiresistive hydrogen sulfide gas sensor. Appl. Phys. Lett. 2009;94:083502.
-
- Ramgir N. S. Sharma P. K. Datta N. Kaur M. Debnath A. K. Aswal D. K. Gupta S. K. Room temperature H2S gas sensor based on Au modified ZnO nanowires. Sens. Actuators, B. 2013;186:718–726.
-
- Li X. Zhang Y. Bhattacharya A. Chu X. F. Liang S. Zeng D. The formaldehyde sensing properties of CdGa2O4 prepared by co-precipitation method. Sens. Actuators, B. 2021;343:129834.
-
- Yang J. Gui Y. Wang Y. He S. NiO/Ti3C2Tx MXene nanocomposites sensor for ammonia gas detection at room temperature. J. Ind. Eng. Chem. 2023;119:476–484.
-
- Chen X. Liu T. Wu R. Yu J. Yin X. Gas sensors based on Pd-decorated and Sb-doped SnO2 for hydrogen detection. J. Ind. Eng. Chem. 2022;115:491–499.
LinkOut - more resources
Full Text Sources

