Novel "on-off" fluorescence sensing for rapid and accurate determination of Cr3+ based on g-CNQDs
- PMID: 37780737
- PMCID: PMC10534202
- DOI: 10.1039/d3ra05091b
Novel "on-off" fluorescence sensing for rapid and accurate determination of Cr3+ based on g-CNQDs
Abstract
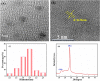
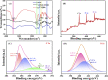
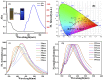
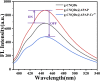
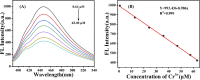
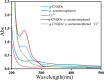
Cr3+ is one of the most essential trace elements in living organisms and plays a vital role in human metabolism. However, both deficiency and excess intake of Cr3+ can be harmful to the human body. Therefore, the quantitative determination of Cr3+ is of great significance in the field of life science. Based on this, in this study, a g-CNQDs@p-acetaminophenol fluorescence sensing system was developed for the quantitative detection of Cr3+ in actual complex samples. G-CNQDs were synthesized with sodium citrate and urea as precursors. The fluorescence signal was enhanced by the synergistic effect between p-acetaminophenol (APAP) and g-CNQDs. The fluorescence quenching phenomenon can be produced when Cr3+ is introduced into the fluorescence-enhanced g-CNQDs@p-acetaminophenol system. An "on-off" fluorescence sensing system was constructed based on g-CNQDs@p-acetaminophenol for the quantitative detection of Cr3+. The experimental data showed a wide linear region in the concentration range of 0.64-63.0 μM, and the detection limit was as low as 0.23 μM. The construction of the sensor system broadens the research field for the practical application of Cr3+.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Fabrication of Fluorescence Turn-off-on Sensor Based on g-C3N4 Quantum Dots and MgFe Layered Double Hydroxide for the Detection of Citrate.J Fluoresc. 2019 May;29(3):719-726. doi: 10.1007/s10895-019-02391-3. Epub 2019 May 16. J Fluoresc. 2019. PMID: 31093827
-
Selective and high-sensitive label-free detection of ascorbic acid by carbon nitride quantum dots with intense fluorescence from lone pair states.Talanta. 2019 May 1;196:530-536. doi: 10.1016/j.talanta.2019.01.003. Epub 2019 Jan 3. Talanta. 2019. PMID: 30683401
-
Switch-on fluorescence sensing of glutathione in food samples based on a graphitic carbon nitride quantum dot (g-CNQD)-Hg²⁺ chemosensor.J Agric Food Chem. 2015 Feb 18;63(6):1747-55. doi: 10.1021/jf505759z. Epub 2015 Feb 9. J Agric Food Chem. 2015. PMID: 25630354
-
Resonance energy transfer based electrochemiluminescence and fluorescence sensing of riboflavin using graphitic carbon nitride quantum dots.Anal Chim Acta. 2017 Jun 22;973:34-42. doi: 10.1016/j.aca.2017.03.041. Epub 2017 Apr 10. Anal Chim Acta. 2017. PMID: 28502425
-
Graphitic carbon nitride quantum dots as an "off-on" fluorescent switch for determination of mercury(II) and sulfide.Mikrochim Acta. 2018 Sep 20;185(10):471. doi: 10.1007/s00604-018-2994-0. Mikrochim Acta. 2018. PMID: 30238322
Cited by
-
Fluorescence/colorimetric dual-signal sensor based on carbon dots and gold nanoparticles for visual quantification of Cr3.Mikrochim Acta. 2024 Sep 3;191(10):571. doi: 10.1007/s00604-024-06645-1. Mikrochim Acta. 2024. PMID: 39223328
References
-
- Tian X.-M. Yao S.-L. Qiu C.-Q. Zheng T.-F. Chen Y.-Q. Huang H. Chen J.-L. Liu S.-J. Wen H.-R. Inorg. Chem. 2020;59:2803–2810. - PubMed
-
- Cheng W. Tang P. He X. Xing X. Liu S. Zhang F. Lu X. Zhong L. Anal. Bioanal. Chem. 2021;413:2951–2960. - PubMed
-
- Tsai M.-J. Liao K.-S. Hsu L.-J. Wu J.-Y. J. Solid State Chem. 2021;304:122564.
-
- Yu Y. e. Wang Y. Yan H. Lu J. Liu H. Li Y. Wang S. Li D. Dou J. Yang L. Inorg. Chem. 2020;59:3828–3837. - PubMed
-
- Paul S. Manna A. Goswami S. Dalton Trans. 2015;44:11805–11810. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

