A deformability-based biochip for precise label-free stratification of metastatic subtypes using deep learning
- PMID: 37780810
- PMCID: PMC10539402
- DOI: 10.1038/s41378-023-00577-1
A deformability-based biochip for precise label-free stratification of metastatic subtypes using deep learning
Abstract
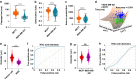
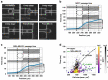
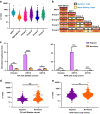
Cellular deformability is a promising biomarker for evaluating the physiological state of cells in medical applications. Microfluidics has emerged as a powerful technique for measuring cellular deformability. However, existing microfluidic-based assays for measuring cellular deformability rely heavily on image analysis, which can limit their scalability for high-throughput applications. Here, we develop a parallel constriction-based microfluidic flow cytometry device and an integrated computational framework (ATMQcD). The ATMQcD framework includes automatic training set generation, multiple object tracking, segmentation, and cellular deformability quantification. The system was validated using cancer cell lines of varying metastatic potential, achieving a classification accuracy of 92.4% for invasiveness assessment and stratifying cancer cells before and after hypoxia treatment. The ATMQcD system also demonstrated excellent performance in distinguishing cancer cells from leukocytes (accuracy = 89.5%). We developed a mechanical model based on power-law rheology to quantify stiffness, which was fitted with measured data directly. The model evaluated metastatic potentials for multiple cancer types and mixed cell populations, even under real-world clinical conditions. Our study presents a highly robust and transferable computational framework for multiobject tracking and deformation measurement tasks in microfluidics. We believe that this platform has the potential to pave the way for high-throughput analysis in clinical applications, providing a powerful tool for evaluating cellular deformability and assessing the physiological state of cells.
Keywords: Bionanoelectronics; Microfluidics.
© Aerospace Information Research Institute, Chinese Academy of Sciences 2023.
Conflict of interest statement
Conflict of interestThe authors declare no competing interests.
Figures




Similar articles
-
Biophysical phenotyping of single cells using a differential multiconstriction microfluidic device with self-aligned 3D electrodes.Biosens Bioelectron. 2019 May 15;133:16-23. doi: 10.1016/j.bios.2019.03.002. Epub 2019 Mar 7. Biosens Bioelectron. 2019. PMID: 30903937
-
Single-Cell Stretching in Viscoelastic Fluids with Electronically Triggered Imaging for Cellular Mechanical Phenotyping.Anal Chem. 2021 Mar 16;93(10):4567-4575. doi: 10.1021/acs.analchem.0c05009. Epub 2021 Mar 4. Anal Chem. 2021. PMID: 33661609
-
Automatic elasticity measurement of single cells using a microfluidic system with real-time image processing.Annu Int Conf IEEE Eng Med Biol Soc. 2023 Jul;2023:1-4. doi: 10.1109/EMBC40787.2023.10340799. Annu Int Conf IEEE Eng Med Biol Soc. 2023. PMID: 38083301
-
Microfluidic deformability cytometry: A review.Talanta. 2023 Jan 1;251:123815. doi: 10.1016/j.talanta.2022.123815. Epub 2022 Aug 6. Talanta. 2023. PMID: 35952505 Review.
-
Shear flow deformability cytometry: A microfluidic method advancing towards clinical use - A review.Anal Chim Acta. 2025 Jun 15;1355:343894. doi: 10.1016/j.aca.2025.343894. Epub 2025 Mar 4. Anal Chim Acta. 2025. PMID: 40274322 Review.
Cited by
-
Mechanical phenotype of cancer cells and its measurement methodology.Anal Sci. 2025 Mar;41(3):185-186. doi: 10.1007/s44211-025-00714-y. Anal Sci. 2025. PMID: 39960617 No abstract available.
-
Neural Network-Enabled Multiparametric Impedance Signal Templating for High throughput Single-Cell Deformability Cytometry Under Viscoelastic Extensional Flows.Small. 2025 Feb;21(5):e2407212. doi: 10.1002/smll.202407212. Epub 2024 Oct 22. Small. 2025. PMID: 39439143 Free PMC article.
-
Editorial Perspective: Advancements in Microfluidics and Biochip Technologies.Micromachines (Basel). 2025 Jan 11;16(1):77. doi: 10.3390/mi16010077. Micromachines (Basel). 2025. PMID: 39858732 Free PMC article.
-
Cancer-on-a-chip for precision cancer medicine.Lab Chip. 2025 Jul 8;25(14):3314-3347. doi: 10.1039/d4lc01043d. Lab Chip. 2025. PMID: 40376718 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
