Preclinical to clinical translation for intervertebral disc repair: Effects of species-specific scale, metabolism, and matrix synthesis rates on cell-based regeneration
- PMID: 37780829
- PMCID: PMC10540833
- DOI: 10.1002/jsp2.1279
Preclinical to clinical translation for intervertebral disc repair: Effects of species-specific scale, metabolism, and matrix synthesis rates on cell-based regeneration
Abstract
Background: A significant hurdle for potential cell-based therapies is the subsequent survival and regenerative capacity of implanted cells. While many exciting developments have demonstrated promise preclinically, cell-based therapies for intervertebral disc (IVD) degeneration fail to translate equivalent clinical efficacy.
Aims: This work aims to ascertain the clinical relevance of both a small and large animal model by experimentally investigating and comparing these animal models to human from the perspective of anatomical scale and their cellular metabolic and regenerative potential.
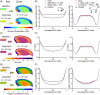
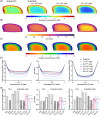
Materials and methods: First, this work experimentally investigated species-specific geometrical scale, native cell density, nutrient metabolism, and matrix synthesis rates for rat, goat, and human disc cells in a 3D microspheroid configuration. Second, these parameters were employed in silico to elucidate species-specific nutrient microenvironments and predict differences in temporal regeneration between animal models.
Results: This work presents in silico models which correlate favorably to preclinical literature in terms of the capabilities of animal regeneration and predict that compromised nutrition is not a significant challenge in small animal discs. On the contrary, it highlights a very fine clinical balance between an adequate cell dose for sufficient repair, through de novo matrix deposition, without exacerbating the human microenvironmental niche.
Discussion: Overall, this work aims to provide a path towards understanding the effect of cell injection number on the nutrient microenvironment and the "time to regeneration" between preclinical animal models and the large human IVD. While these findings help to explain failed translation of promising preclinical data and the limited results emerging from clinical trials at present, they also enable the research field and clinicians to manage expectations on cell-based regeneration.
Conclusion: Ultimately, this work provides a platform to inform the design of clinical trials, and as computing power and software capabilities increase in the future, it is conceivable that generation of patient-specific models could be used for patient assessment, as well as pre- and intraoperative planning.
Keywords: animal models; cell therapies; in silico; metabolism; regeneration.
© 2023 The Authors. JOR Spine published by Wiley Periodicals LLC on behalf of Orthopaedic Research Society.
Conflict of interest statement
Conor T. Buckley is an Editorial Board member of JOR Spine and co‐author of this article. They were excluded from editorial decision‐making related to the acceptance of this article for publication in the journal.
Figures




Similar articles
-
Investigating the physiological relevance of ex vivo disc organ culture nutrient microenvironments using in silico modeling and experimental validation.JOR Spine. 2021 Mar 2;4(2):e1141. doi: 10.1002/jsp2.1141. eCollection 2021 Jun. JOR Spine. 2021. PMID: 34337330 Free PMC article.
-
Consolidating and re-evaluating the human disc nutrient microenvironment.JOR Spine. 2022 Feb 1;5(1):e1192. doi: 10.1002/jsp2.1192. eCollection 2022 Mar. JOR Spine. 2022. PMID: 35386756 Free PMC article.
-
Low energy extracorporeal shock wave therapy combined with low tension traction can better reshape the microenvironment in degenerated intervertebral disc regeneration and repair.Spine J. 2021 Jan;21(1):160-177. doi: 10.1016/j.spinee.2020.08.004. Epub 2020 Aug 13. Spine J. 2021. PMID: 32800896
-
Evaluation of Tissue Engineering Approaches for Intervertebral Disc Regeneration in Relevant Animal Models.ACS Appl Bio Mater. 2021 Nov 15;4(11):7721-7737. doi: 10.1021/acsabm.1c00500. Epub 2021 Oct 15. ACS Appl Bio Mater. 2021. PMID: 35006757 Review.
-
The effects of microenvironment in mesenchymal stem cell-based regeneration of intervertebral disc.Spine J. 2013 Mar;13(3):352-62. doi: 10.1016/j.spinee.2012.12.005. Epub 2013 Jan 20. Spine J. 2013. PMID: 23340343 Review.
Cited by
-
pH: A major player in degenerative intervertebral disks.JOR Spine. 2024 Dec 18;7(4):e70025. doi: 10.1002/jsp2.70025. eCollection 2024 Dec. JOR Spine. 2024. PMID: 39703199 Free PMC article. Review.
-
Getting to the Core: Exploring the Embryonic Development from Notochord to Nucleus Pulposus.J Dev Biol. 2024 Jul 3;12(3):18. doi: 10.3390/jdb12030018. J Dev Biol. 2024. PMID: 39051200 Free PMC article. Review.
-
In silico modeling the potential clinical effect of growth factor treatment on the metabolism of human nucleus pulposus cells.JOR Spine. 2024 Jul 31;7(3):e1352. doi: 10.1002/jsp2.1352. eCollection 2024 Sep. JOR Spine. 2024. PMID: 39092165 Free PMC article.
-
Banked Primary Progenitor Cells for Allogeneic Intervertebral Disc (IVD) Therapy: Preclinical Qualification and Functional Optimization within a Cell Spheroid Formulation Process.Pharmaceutics. 2024 Sep 29;16(10):1274. doi: 10.3390/pharmaceutics16101274. Pharmaceutics. 2024. PMID: 39458605 Free PMC article.
-
Intradiscal administration of autologous platelet-rich plasma in patients with Modic type 1 associated low back pain: A prospective pilot study.JOR Spine. 2024 Mar 18;7(1):e1320. doi: 10.1002/jsp2.1320. eCollection 2024 Mar. JOR Spine. 2024. PMID: 38500785 Free PMC article.
References
LinkOut - more resources
Full Text Sources

