Significance of CMV reactivation in non-allogeneic stem cell transplant patients with cancers: experience of single tertiary care cancer institute
- PMID: 37780907
- PMCID: PMC10533462
- DOI: 10.1007/s13337-023-00839-6
Significance of CMV reactivation in non-allogeneic stem cell transplant patients with cancers: experience of single tertiary care cancer institute
Abstract
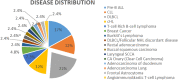
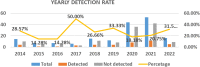
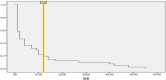
CMV reactivation is rare in hematological as well as solid organ malignancies in non-allogeneic stem cell transplant settings. An increasing number of patients undergoing active treatment or follow-up and diagnosed with CMV reactivation in recent years prompted us to investigate the risk factors and outcomes of CMV reactivation or disease. This was a hospital-based retrospective study that included 174 cancer patients suspected of CMV reactivation. Among them, forty-one tested positive for CMV viremia. The risk factors for CMV reactivation included the use of steroids in 78% of patients, active cancer in 43.9%, use of a monoclonal antibody rituximab in 31.7%, a history of radiation in 26.8%, and autologous stem cell transplant in 12% of patients. The median age was 36 years, and the most common clinical feature was fever (58.5%; n = 24), followed by GI symptoms (12.1%; n = 5), respiratory symptoms (14.6%; n = 6), cytopenia (7.3%; n = 3), and visual/neurological symptoms (4.8%; n = 2). The mean CMV viral load was 37,332 copies/ml (range: 75.00-633,000.00 copies/ml). Nineteen patients received CMV treatment with an average treatment duration of 81.5 days. The median overall survival was 2 months, with 12.0% of patients alive at 5 years. CMV reactivation is associated with significant morbidity and mortality. We recommend vigilant monitoring of CMV-related symptoms, with a low threshold for testing and treatment, for patients with multiple risk factors for CMV reactivation.
Keywords: Cytomegalovirus; Non-allogenic transplant; Reactivation.
© The Author(s), under exclusive licence to Indian Virological Society 2023. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflict of interestAll authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Figures
References
LinkOut - more resources
Full Text Sources

