Vascular endothelial cell-specific disruption of the profilin1 gene leads to severe multiorgan pathology and inflammation causing mortality
- PMID: 37781098
- PMCID: PMC10541205
- DOI: 10.1093/pnasnexus/pgad305
Vascular endothelial cell-specific disruption of the profilin1 gene leads to severe multiorgan pathology and inflammation causing mortality
Abstract
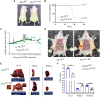
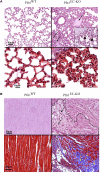
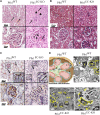
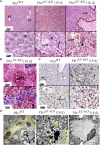
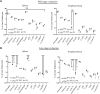
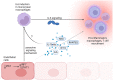
Actin-binding protein Profilin1 is an important regulator of actin cytoskeletal dynamics in cells and critical for embryonic development in higher eukaryotes. The objective of the present study was to examine the consequence of loss-of-function of Pfn1 in vascular endothelial cells (ECs) in vivo. We utilized a mouse model engineered for tamoxifen-inducible biallelic inactivation of the Pfn1 gene selectively in EC (Pfn1EC-KO). Widespread deletion of EC Pfn1 in adult mice leads to severe health complications presenting overt pathologies (endothelial cell death, infarct, and fibrosis) in major organ systems and evidence for inflammatory infiltrates, ultimately compromising the survival of animals within 3 weeks of gene ablation. Mice deficient in endothelial Pfn1 exhibit selective bias toward the proinflammatory myeloid-derived population of immune cells, a finding further supported by systemic elevation of proinflammatory cytokines. We further show that triggering Pfn1 depletion not only directly upregulates proinflammatory cytokine/chemokine gene expression in EC but also potentiates the paracrine effect of EC on proinflammatory gene expression in macrophages. Consistent with these findings, we provide further evidence for increased activation of Interferon Regulatory Factor 7 (IRF7) and STAT1 in EC when depleted of Pfn1. Collectively, these findings for the first time demonstrate a prominent immunological consequence of loss of endothelial Pfn1 and an indispensable role of endothelial Pfn1 in mammalian survival unlike tolerable phenotypes of Pfn1 loss in other differentiated cell types.
Keywords: actin; endothelial cells; inflammation; macrophage; profilin; vascular.
© The Author(s) 2023. Published by Oxford University Press on behalf of National Academy of Sciences.
Figures


References
-
- Pandey DK, Chaudhary B. 2017. Evolutionary expansion and structural functionalism of the ancient family of profilin proteins. Gene. 626:70–86. - PubMed
-
- Witke W. 2004. The role of profilin complexes in cell motility and other cellular processes. Trends Cell Biol. 14:461–469. - PubMed
-
- Ding Z, Lambrechts A, Parepally M, Roy P. 2006. Silencing profilin-1 inhibits endothelial cell proliferation, migration and cord morphogenesis. J Cell Sci. 119:4127–4137. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

